Influences on participant reporting in the World Health Organisation drugs exposure pregnancy registry; a qualitative study
- PMID: 25367130
- PMCID: PMC4229602
- DOI: 10.1186/s12913-014-0525-1
Influences on participant reporting in the World Health Organisation drugs exposure pregnancy registry; a qualitative study
Abstract
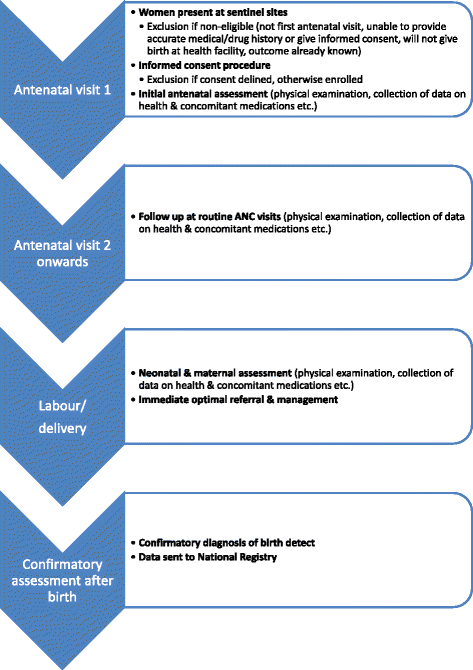
Background: The World Health Organisation has designed a pregnancy registry to investigate the effect of maternal drug use on pregnancy outcomes in resource-limited settings. In this sentinel surveillance system, detailed health and drug use data are prospectively collected from the first antenatal clinic visit until delivery. Over and above other clinical records, the registry relies on accurate participant reports about the drugs they use. Qualitative methods were incorporated into a pilot registry study during 2010 and 2011 to examine barriers to women reporting these drugs and other exposures at antenatal clinics, and how they might be overcome.
Methods: Twenty-seven focus group discussions were conducted in Ghana, Kenya and Uganda with a total of 208 women either enrolled in the registry or from its source communities. A question guide was designed to uncover the types of exposure data under- or inaccurately reported at antenatal clinics, the underlying reasons, and how women prefer to be asked questions. Transcripts were analysed thematically.
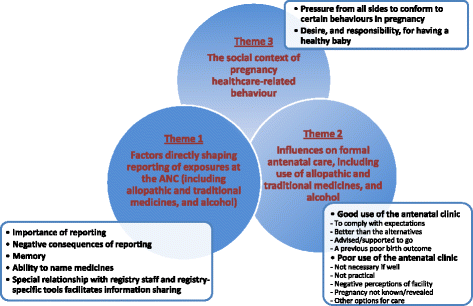
Results: Women said it was important for them to report everything they had used during pregnancy. However, they expressed reservations about revealing their consumption of traditional, over-the-counter medicines and alcohol to antenatal staff because of anticipated negative reactions. Some enrolled participants' improved relationship with registry staff facilitated information sharing and the registry tools helped overcome problems with recall and naming of medicines. Decisions about where women sought care, which influenced medicines used and antenatal clinic attendance, were influenced by pressure within and outside of the formal healthcare system to conform to conflicting behaviours. Conversations also reflected women's responsibilities for producing a healthy baby.
Conclusions: Women in this study commonly take traditional medicines in pregnancy, and to a lesser extent over-the-counter medicines and alcohol. The World Health Organisation pregnancy registry shows potential to enhance their reporting of these substances at the antenatal clinic. However, more work is needed to find optimal techniques for eliciting accurate reports, especially where the detail of constituents may never be known. It will also be important to find ways of sustaining such drug exposure surveillance systems in busy antenatal clinics.
Figures
Similar articles
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Provision and uptake of routine antenatal services: a qualitative evidence synthesis.Cochrane Database Syst Rev. 2019 Jun 12;6(6):CD012392. doi: 10.1002/14651858.CD012392.pub2. Cochrane Database Syst Rev. 2019. PMID: 31194903 Free PMC article.
-
Losing women along the path to safe motherhood: why is there such a gap between women's use of antenatal care and skilled birth attendance? A mixed methods study in northern Uganda.BMC Pregnancy Childbirth. 2015 Nov 4;15:287. doi: 10.1186/s12884-015-0695-9. BMC Pregnancy Childbirth. 2015. PMID: 26538084 Free PMC article.
-
Protocol for a drugs exposure pregnancy registry for implementation in resource-limited settings.BMC Pregnancy Childbirth. 2012 Sep 3;12:89. doi: 10.1186/1471-2393-12-89. BMC Pregnancy Childbirth. 2012. PMID: 22943425 Free PMC article.
-
'I fear my partner will abandon me': the intersection of late initiation of antenatal care in pregnancy and poor ART adherence among women living with HIV in South Africa and Uganda.BMC Pregnancy Childbirth. 2022 Jul 15;22(1):566. doi: 10.1186/s12884-022-04896-5. BMC Pregnancy Childbirth. 2022. PMID: 35840939 Free PMC article.
Cited by
-
Inclusion of pregnant women in antiretroviral drug research: what is needed to move forwards?J Int AIDS Soc. 2019 Sep;22(9):e25372. doi: 10.1002/jia2.25372. J Int AIDS Soc. 2019. PMID: 31529598 Free PMC article.
-
Discovering Cohorts of Pregnant Women From Social Media for Safety Surveillance and Analysis.J Med Internet Res. 2017 Oct 30;19(10):e361. doi: 10.2196/jmir.8164. J Med Internet Res. 2017. PMID: 29084707 Free PMC article.
-
Incidence, determinants and outcomes of pregnancy-associated hepatitis B flares: A regional hospital-based cohort study.Liver Int. 2018 May;38(5):813-820. doi: 10.1111/liv.13594. Epub 2017 Sep 30. Liver Int. 2018. PMID: 28941137 Free PMC article.
-
Assessing the value of Western Cape Provincial Government health administrative data and electronic pharmacy records in ascertaining medicine use during pregnancy.S Afr Med J. 2018 Apr 25;108(5):439-443. doi: 10.7196/SAMJ.2018.v108i5.12879. S Afr Med J. 2018. PMID: 29843860 Free PMC article.
-
Determining antenatal medicine exposures in South African women: a comparison of three methods of ascertainment.BMC Pregnancy Childbirth. 2022 Jun 3;22(1):466. doi: 10.1186/s12884-022-04765-1. BMC Pregnancy Childbirth. 2022. PMID: 35658841 Free PMC article.
References
-
- National policy on traditional medicine and regulation of herbal medicines: Report of a WHO Global Survey. Geneva: 2005. http://apps.who.int/medicinedocs/en/d/Js7916e/.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical