The development and validation of a disease-specific quality of life measure in hyperhidrosis: the Hyperhidrosis Quality of Life Index (HidroQOL©)
- PMID: 25367139
- PMCID: PMC4366556
- DOI: 10.1007/s11136-014-0825-2
The development and validation of a disease-specific quality of life measure in hyperhidrosis: the Hyperhidrosis Quality of Life Index (HidroQOL©)
Abstract
Purpose: To develop and validate a new disease-specific quality of life measure in hyperhidrosis for use in both routine clinical practice and clinical research.
Methods: Interviews and focus group discussions with hyperhidrosis patients, reported elsewhere, provided the content for the measure validated in this study (n = 71). A panel of dermatologists (n = 5) and patients (n = 7) carried out content validation. Further, item reduction and the initial construct validation were carried out in a cross-sectional study (n = 595), using the unidimensional Rasch analysis and exploratory factor analysis. Subsequently, the construct validity, reliability and responsiveness of the revised measure were assessed in a longitudinal study (n = 260). Data collection for the item reduction and the final validation phases was entirely carried out online.
Results: The expert panels judged the HidroQoL as content valid. Rasch analysis supported the revision of response options from five to three. Following removal of misfitting items, a set of 15 items showed optimal fit to the model (chi-squared statistic = 159.64, p = 0.07). Three additional items were retained on consideration of their importance to patients, resulting in an 18-item instrument. The items were grouped into two subscales, daily life activities and psychosocial life domains, based on results of the factor analysis. In subsequent construct validation, the HidroQoL correlated with the DLQI (r s = 0.6, p < 0.01). Reliability was high (internal consistency, Cronbach's alpha: overall scale = 0.9; test-retest reliability, Intra-class correlation = 0.9). The HidroQoL scores were sensitive to change in patients' disease severity (score change from baseline to follow-up after 15-35 days, Cohen's ES = 0.47).
Conclusion: This study has provided the initial evidence supporting measurement properties and the use of the HidroQoL instrument in both routine clinical practice and in research, for assessing quality of life impacts in hyperhidrosis.
Figures
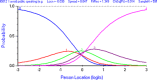
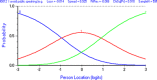

Similar articles
-
Hyperhidrosis quality of life index (HidroQoL): further validation by applying classical test theory and item response theory using data from a phase III clinical trial.J Patient Rep Outcomes. 2023 Jun 6;7(1):55. doi: 10.1186/s41687-023-00596-6. J Patient Rep Outcomes. 2023. PMID: 37280417 Free PMC article. Clinical Trial.
-
Hyperhidrosis Quality of Life Index (HidroQoL©): further validation and clinical application in patients with axillary hyperhidrosis using data from a phase III randomized controlled trial.Br J Dermatol. 2021 Mar;184(3):473-481. doi: 10.1111/bjd.19300. Epub 2020 Sep 1. Br J Dermatol. 2021. PMID: 32510573 Clinical Trial.
-
The Creation of the Arabic Version of the Hyperhidrosis Quality of Life Index (HidroQoL©) with Validation and Cross-Cultural Adaptation.Clin Cosmet Investig Dermatol. 2025 Jan 25;18:251-263. doi: 10.2147/CCID.S498688. eCollection 2025. Clin Cosmet Investig Dermatol. 2025. PMID: 39881852 Free PMC article.
-
The development and validation of the Cluster Headache Quality of life scale (CHQ).J Headache Pain. 2016 Dec;17(1):79. doi: 10.1186/s10194-016-0674-1. Epub 2016 Sep 5. J Headache Pain. 2016. PMID: 27596922 Free PMC article. Review.
-
Hyperhidrosis quality of life measures: review and patient perspective.J Dermatolog Treat. 2019 May;30(3):303-308. doi: 10.1080/09546634.2018.1506080. Epub 2018 Sep 12. J Dermatolog Treat. 2019. PMID: 30051732 Review.
Cited by
-
Continuous evaluation of exosomatic electrodermal activity in patients with primary palmoplantar hyperhidrosis.Einstein (Sao Paulo). 2024 Dec 9;22:eAO1152. doi: 10.31744/einstein_journal/2024AO1152. eCollection 2024. Einstein (Sao Paulo). 2024. PMID: 39661855 Free PMC article.
-
Development and validation of the Axillary Sweating Daily Diary: a patient-reported outcome measure to assess axillary sweating severity.J Patient Rep Outcomes. 2019 Sep 5;3(1):59. doi: 10.1186/s41687-019-0148-8. J Patient Rep Outcomes. 2019. PMID: 31486951 Free PMC article.
-
Impact of primary hyperhidrosis on patients' quality of life in Damietta governorates.Arch Dermatol Res. 2025 Mar 12;317(1):551. doi: 10.1007/s00403-025-04034-z. Arch Dermatol Res. 2025. PMID: 40072576
-
Arabic Translation, Cultural Adaptation, and Validation of the Hyperhidrosis Disease Severity Scale (Ar-HDSS).Healthcare (Basel). 2025 Feb 12;13(4):397. doi: 10.3390/healthcare13040397. Healthcare (Basel). 2025. PMID: 39997272 Free PMC article.
-
Systemic effect of sympathectomy in the treatment of localized hyperhidrosis.Updates Surg. 2025 Mar 10. doi: 10.1007/s13304-025-02163-8. Online ahead of print. Updates Surg. 2025. PMID: 40064815
References
-
- Solish N. Assessing hyperhidrosis disease severity and impact on quality of life. Cutis. 2006;77(Suppl. 5):17–27.
-
- Solish N, Bertucci V, Dansereau A, Hong HC, Lynde C, Lupin M, et al. A comprehensive approach to the recognition, diagnosis, and severity-based treatment of focal hyperhidrosis: Recommendations of the Canadian Hyperhidrosis Advisory Committee. Dermatologic Surgery. 2007;33(8):908–923. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

