The roles and regulation of Polycomb complexes in neural development
- PMID: 25367430
- PMCID: PMC4286515
- DOI: 10.1007/s00441-014-2011-9
The roles and regulation of Polycomb complexes in neural development
Abstract
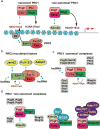
In the developing mammalian nervous system, common progenitors integrate both cell extrinsic and intrinsic regulatory programs to produce distinct neuronal and glial cell types as development proceeds. This spatiotemporal restriction of neural progenitor differentiation is enforced, in part, by the dynamic reorganization of chromatin into repressive domains by Polycomb repressive complexes, effectively limiting the expression of fate-determining genes. Here, we review the distinct roles that Polycomb repressive complexes play during neurogenesis and gliogenesis, while also highlighting recent work describing the molecular mechanisms that govern their dynamic activity in neural development. Further investigation of the way in which Polycomb complexes are regulated in neural development will enable more precise manipulation of neural progenitor differentiation facilitating the efficient generation of specific neuronal and glial cell types for many biological applications.
Figures


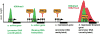
References
-
- Aprea J, Prenninger S, Dori M, Ghosh T, Monasor LS, Wessendorf E, Zocher S, Massalini S, Alexopoulou D, Lesche M, Dahl A, Groszer M, Hiller M, Calegari F. Transcriptome sequencing during mouse brain development identifies long non-coding RNAs functionally involved in neurogenic commitment. EMBO J. 2013;32(24):3145–3160. - PMC - PubMed
-
- Armstrong L. Epigenetic control of embryonic stem cell differentiation. Stem Cell Rev. 2012;8(1):67–77. - PubMed
-
- Arney KL, Fisher AG. Epigenetic aspects of differentiation. J Cell Sci. 2004;117(Pt 19):4355–4363. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

