Stand-up exercise training facilitates muscle recovery from disuse atrophy by stimulating myogenic satellite cell proliferation in mice
- PMID: 25367692
- PMCID: PMC4255801
- DOI: 10.14814/phy2.12185
Stand-up exercise training facilitates muscle recovery from disuse atrophy by stimulating myogenic satellite cell proliferation in mice
Abstract
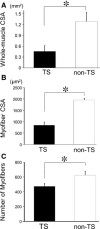
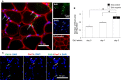
Determining the cellular and molecular recovery processes in inactivity - or unloading -induced atrophied muscles should improve rehabilitation strategies. We assessed the effects of stand-up exercise (SE) training on the recovery of atrophied skeletal muscles in male mice. Mice were trained to stand up and press an elevated lever in response to a light-tone cue preceding an electric foot shock and then subjected to tail suspension (TS) for 2 weeks to induce disuse atrophy in hind limb muscles. After release from TS, mice were divided into SE-trained (SE cues: 25 times per set, two sets per day) and non-SE-trained groups. Seven days after the training, average myofiber cross-sectional area (CSA) of the soleus muscle was significantly greater in the SE-trained group than in the non-SE-trained group (1843 ± 194 μm(2) vs. 1315 ± 153 μm(2)). Mean soleus muscle CSA in the SE trained group was not different from that in the CON group subjected to neither TS nor SE training (2005 ± 196 μm(2)), indicating that SE training caused nearly complete recovery from muscle atrophy. The number of myonuclei per myofiber was increased by ~60% in the SE-trained group compared with the non-SE-trained and CON groups (0.92 ± 0.03 vs. 0.57 ± 0.03 and 0.56 ± 0.11, respectively). The number of proliferating myonuclei, identified by 5-ethynyl-2'-deoxyuridine staining, increased within the first few days of SE training. Thus, it is highly likely that myogenic satellite cells proliferated rapidly in atrophied muscles in response to SE training and fused with existing myofibers to reestablish muscle mass.
Keywords: 5‐ethynyl‐2’‐deoxyuridine; myogenic satellite cells; myonuclei; operant conditioning; stand‐up exercise.
© 2014 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of the American Physiological Society and The Physiological Society.
Figures


References
-
- Antonio J., Gonyea W. J. 1993a. Skeletal muscle fiber hyperplasia. Med. Sci. Sports Exerc.; 25:1333-1345. - PubMed
-
- Antonio J., Gonyea W. J. 1993b. Progressive stretch overload of skeletal muscle results in hypertrophy before hyperplasia. J. Appl. Physiol.; 75:1263-1271. - PubMed
-
- Bischoff R. 1989. Analysis of muscle regeneration using single myofibers in culture. Med. Sci. Sports Exerc.; 215 Suppl:S164-S172. - PubMed
-
- Bruusgaard J. C., Egner I. M., Larsen T. K., Dupre‐Aucouturier S., Desplanches D., Gundersen K. 2012. No change in myonuclear number during muscle unloading and reloading. J. Appl. Physiol.; 113:290-296. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

