How to make a heart valve: from embryonic development to bioengineering of living valve substitutes
- PMID: 25368013
- PMCID: PMC4208706
- DOI: 10.1101/cshperspect.a013912
How to make a heart valve: from embryonic development to bioengineering of living valve substitutes
Abstract
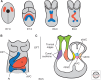
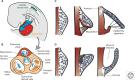
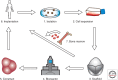
Cardiac valve disease is a significant cause of ill health and death worldwide, and valve replacement remains one of the most common cardiac interventions in high-income economies. Despite major advances in surgical treatment, long-term therapy remains inadequate because none of the current valve substitutes have the potential for remodeling, regeneration, and growth of native structures. Valve development is coordinated by a complex interplay of signaling pathways and environmental cues that cause disease when perturbed. Cardiac valves develop from endocardial cushions that become populated by valve precursor mesenchyme formed by an epithelial-mesenchymal transition (EMT). The mesenchymal precursors, subsequently, undergo directed growth, characterized by cellular compartmentalization and layering of a structured extracellular matrix (ECM). Knowledge gained from research into the development of cardiac valves is driving exploration into valve biomechanics and tissue engineering directed at creating novel valve substitutes endowed with native form and function.
Copyright © 2014 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures


References
-
- Abu-Issa R, Kirby ML. 2007. Heart field: From mesoderm to heart tube. Annu Rev Cell Dev Biol 23: 45–68 - PubMed
-
- Aikawa E, Whittaker P, Farber M, Mendelson K, Padera RF, Aikawa M, Schoen FJ. 2006. Human semilunar cardiac valve remodeling by activated cells from fetus to adult: Implications for postnatal adaptation, pathology, and tissue engineering. Circulation 113: 1344–1352 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
