Epigenetic changes in bone marrow progenitor cells influence the inflammatory phenotype and alter wound healing in type 2 diabetes
- PMID: 25368099
- PMCID: PMC4375075
- DOI: 10.2337/db14-0872
Epigenetic changes in bone marrow progenitor cells influence the inflammatory phenotype and alter wound healing in type 2 diabetes
Abstract
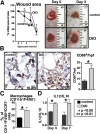
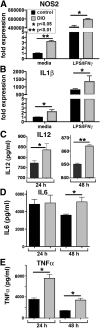
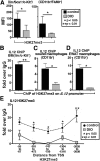
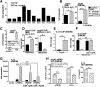
Classically activated (M1) macrophages are known to play a role in the development of chronic inflammation associated with impaired wound healing in type 2 diabetes (T2D); however, the mechanism responsible for the dominant proinflammatory (M1) macrophage phenotype in T2D wounds is unknown. Since epigenetic enzymes can direct macrophage phenotypes, we assessed the role of histone methylation in bone marrow (BM) stem/progenitor cells in the programming of macrophages toward a proinflammatory phenotype. We have found that a repressive histone methylation mark, H3K27me3, is decreased at the promoter of the IL-12 gene in BM progenitors and this epigenetic signature is passed down to wound macrophages in a murine model of glucose intolerance (diet-induced obese). These epigenetically "preprogrammed" macrophages result in poised macrophages in peripheral tissue and negatively impact wound repair. We found that in diabetic conditions the H3K27 demethylase Jmjd3 drives IL-12 production in macrophages and that IL-12 production can be modulated by inhibiting Jmjd3. Using human T2D tissue and murine models, we have identified a previously unrecognized mechanism by which macrophages are programmed toward a proinflammatory phenotype, establishing a pattern of unrestrained inflammation associated with nonhealing wounds. Hence, histone demethylase inhibitor-based therapy may represent a novel treatment option for diabetic wounds.
© 2015 by the American Diabetes Association. Readers may use this article as long as the work is properly cited, the use is educational and not for profit, and the work is not altered.
Figures


Comment in
-
Diabetes: Epigenetic changes lead to impaired wound healing in patients with T2DM.Nat Rev Endocrinol. 2015 Feb;11(2):65. doi: 10.1038/nrendo.2014.207. Epub 2014 Nov 25. Nat Rev Endocrinol. 2015. PMID: 25421373 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

