Huntingtin is required for ER-to-Golgi transport and for secretory vesicle fusion at the plasma membrane
- PMID: 25368120
- PMCID: PMC4257002
- DOI: 10.1242/dmm.017368
Huntingtin is required for ER-to-Golgi transport and for secretory vesicle fusion at the plasma membrane
Abstract
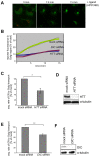
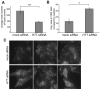
Huntingtin is a large membrane-associated scaffolding protein that associates with endocytic and exocytic vesicles and modulates their trafficking along cytoskeletal tracks. Although the progression of Huntington's disease is linked to toxic accumulation of mutant huntingtin protein, loss of wild-type huntingtin function might also contribute to neuronal cell death, but its precise function is not well understood. Therefore, we investigated the molecular role of huntingtin in exocytosis and observed that huntingtin knockdown in HeLa cells causes a delay in endoplasmic reticulum (ER)-to-Golgi transport and a reduction in the number of cargo vesicles leaving the trans-Golgi network. In addition, we found that huntingtin is required for secretory vesicle fusion at the plasma membrane. Similar defects in the early exocytic pathway were observed in primary fibroblasts from homozygous Htt(140Q/140Q) knock-in mice, which have the expansion inserted into the mouse huntingtin gene so lack wild-type huntingtin expression. Interestingly, heterozygous fibroblasts from a Huntington's disease patient with a 180Q expansion displayed no obvious defects in the early secretory pathway. Thus, our results highlight the requirement for wild-type huntingtin at distinct steps along the secretory pathway.
Keywords: ER; Exocytosis; Golgi; Huntingtin; Vesicle fusion.
© 2014. Published by The Company of Biologists Ltd.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

