Directed evolution of an ultrastable carbonic anhydrase for highly efficient carbon capture from flue gas
- PMID: 25368146
- PMCID: PMC4246266
- DOI: 10.1073/pnas.1411461111
Directed evolution of an ultrastable carbonic anhydrase for highly efficient carbon capture from flue gas
Abstract
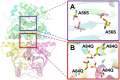
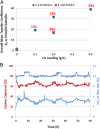
Carbonic anhydrase (CA) is one of nature's fastest enzymes and can dramatically improve the economics of carbon capture under demanding environments such as coal-fired power plants. The use of CA to accelerate carbon capture is limited by the enzyme's sensitivity to the harsh process conditions. Using directed evolution, the properties of a β-class CA from Desulfovibrio vulgaris were dramatically enhanced. Iterative rounds of library design, library generation, and high-throughput screening identified highly stable CA variants that tolerate temperatures of up to 107 °C in the presence of 4.2 M alkaline amine solvent at pH >10.0. This increase in thermostability and alkali tolerance translates to a 4,000,000-fold improvement over the natural enzyme. At pilot scale, the evolved catalyst enhanced the rate of CO2 absorption 25-fold compared with the noncatalyzed reaction.
Keywords: carbon capture; carbonic anhydrase; directed evolution.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Rochelle GT. Amine scrubbing for CO2 capture. Science. 2009;325(5948):1652–1654. - PubMed
-
- Lin LC, et al. In silico screening of carbon-capture materials. Nat Mater. 2012;11(7):633–641. - PubMed
-
- Chu S, Majumdar A. Opportunities and challenges for a sustainable energy future. Nature. 2012;488(7411):294–303. - PubMed
-
- Oexmann J, Kather A. Minimising the regeneration heat duty of post-combustion CO2 capture by wet chemical absorption: The misguided focus on low heat of absorption solvents. Int J Greenh Gas Control. 2010;4(1):36–43.
-
- Blais R, Rogers P. 2003. Process and apparatus for the treatment of carbon dioxide with carbonic anhydrase. US Patent 09/424,852:US6524843.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

