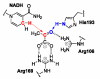
The enzymatic reaction catalyzed by lactate dehydrogenase exhibits one dominant reaction path
- PMID: 25368440
- PMCID: PMC4215548
- DOI: 10.1016/j.chemphys.2014.02.018
The enzymatic reaction catalyzed by lactate dehydrogenase exhibits one dominant reaction path
Abstract
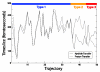
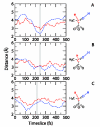
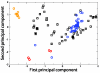
Enzymes are the most efficient chemical catalysts known, but the exact nature of chemical barrier crossing in enzymes is not fully understood. Application of transition state theory to enzymatic reactions indicates that the rates of all possible reaction paths, weighted by their relative probabilities, must be considered in order to achieve an accurate calculation of the overall rate. Previous studies in our group have shown a single mechanism for enzymatic barrier passage in human heart lactate dehydrogenase (LDH). To ensure that this result was not due to our methodology insufficiently sampling reactive phase space, we implement high-perturbation transition path sampling in both microcanonical and canonical regimes for the reaction catalyzed by human heart LDH. We find that, although multiple, distinct paths through reactive phase space are possible for this enzymatic reaction, one specific reaction path is dominant. Since the frequency of these paths in a canonical ensemble is inversely proportional to the free energy barriers separating them from other regions of phase space, we conclude that the rarer reaction paths are likely to have a negligible contribution. Furthermore, the non-dominate reaction paths correspond to altered reactive conformations and only occur after multiple steps of high perturbation, suggesting that these paths may be the result of non-biologically significant changes to the structure of the enzymatic active site.
Keywords: enzymatic catalysis; lactate dehydrogenase; reaction coordinate; transition path sampling.
Figures

References
-
- Pauling L. Chemical achievement and hope for the future. Am. Sci. 1948;36:51–58. - PubMed
-
- Schultz PG. Catalytic Antibodies. Angew. Chem. Int. Ed. 1989;28:1283–1295.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
