A unifying computational framework for stability and flexibility of arousal
- PMID: 25368557
- PMCID: PMC4202806
- DOI: 10.3389/fnsys.2014.00192
A unifying computational framework for stability and flexibility of arousal
Abstract
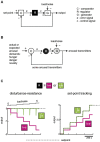
Arousal and consciousness flexibly adjust to salient cues, but remain stable despite noise and disturbance. Diverse, highly interconnected neural networks govern the underlying transitions of behavioral state; these networks are robust but very complex. Frameworks from systems engineering provide powerful tools for understanding functional logic behind component complexity. From a general systems viewpoint, a minimum of three communicating control modules may enable flexibility and stability to coexist. Comparators would subtract current arousal from desired arousal, producing an error signal. Regulators would compute control signals from this error. Generators would convert control signals into arousal, which is fed back to comparators, to make the system noise-proof through self-correction. Can specific neurons correspond to these control elements? To explore this, here we consider the brain-wide orexin/hypocretin network, which is experimentally established to be vital for flexible and stable arousal. We discuss whether orexin neurons may act as comparators, and their target neurons as regulators and generators. Experiments are proposed for testing such predictions, based on computational simulations showing that comparators, regulators, and generators have distinct temporal signatures of activity. If some regulators integrate orexin-communicated errors, robust arousal control may be achieved via integral feedback (a basic engineering strategy for tracking a set-point despite noise). An integral feedback view also suggests functional roles for specific molecular aspects, such as differing life-spans of orexin peptides. The proposed framework offers a unifying logic for molecular, cellular, and network details of arousal systems, and provides insight into behavioral state transitions, complex behavior, and bases for disease.
Keywords: arousal; computation; control; histamine; hypocretin; hypothalamus; narcolepsy; orexin.
Figures


Similar articles
-
Orexin/Hypocretin and Organizing Principles for a Diversity of Wake-Promoting Neurons in the Brain.Curr Top Behav Neurosci. 2017;33:51-74. doi: 10.1007/7854_2016_45. Curr Top Behav Neurosci. 2017. PMID: 27830577 Free PMC article. Review.
-
Plasticity in neurons synthesizing wake/arousal promoting hormone hypocretin/orexin.Vitam Horm. 2012;89:35-59. doi: 10.1016/B978-0-12-394623-2.00003-2. Vitam Horm. 2012. PMID: 22640607 Review.
-
A vertebrate family without a functional Hypocretin/Orexin arousal system.Curr Biol. 2024 Apr 8;34(7):1532-1540.e4. doi: 10.1016/j.cub.2024.02.022. Epub 2024 Mar 14. Curr Biol. 2024. PMID: 38490200
-
Convergent excitation of dorsal raphe serotonin neurons by multiple arousal systems (orexin/hypocretin, histamine and noradrenaline).J Neurosci. 2002 Oct 15;22(20):8850-9. doi: 10.1523/JNEUROSCI.22-20-08850.2002. J Neurosci. 2002. PMID: 12388591 Free PMC article.
-
Function and dysfunction of hypocretin/orexin: an energetics point of view.Annu Rev Neurosci. 2014;37:101-16. doi: 10.1146/annurev-neuro-071013-013855. Epub 2014 Apr 24. Annu Rev Neurosci. 2014. PMID: 24821311 Review.
Cited by
-
Neuropeptides as Primary Mediators of Brain Circuit Connectivity.Front Neurosci. 2021 Mar 11;15:644313. doi: 10.3389/fnins.2021.644313. eCollection 2021. Front Neurosci. 2021. PMID: 33776641 Free PMC article. Review.
-
Hubs and spokes of the lateral hypothalamus: cell types, circuits and behaviour.J Physiol. 2016 Nov 15;594(22):6443-6462. doi: 10.1113/JP271946. Epub 2016 Jul 19. J Physiol. 2016. PMID: 27302606 Free PMC article. Review.
-
Sleep, recovery, and metaregulation: explaining the benefits of sleep.Nat Sci Sleep. 2015 Dec 17;7:171-84. doi: 10.2147/NSS.S54036. eCollection 2015. Nat Sci Sleep. 2015. PMID: 26719733 Free PMC article.
-
Orexin/Hypocretin and MCH Neurons: Cognitive and Motor Roles Beyond Arousal.Front Neurosci. 2021 Mar 22;15:639313. doi: 10.3389/fnins.2021.639313. eCollection 2021. Front Neurosci. 2021. PMID: 33828450 Free PMC article. Review.
-
Ongoing behavioral state information signaled in the lateral habenula guides choice flexibility in freely moving rats.Front Behav Neurosci. 2015 Nov 4;9:295. doi: 10.3389/fnbeh.2015.00295. eCollection 2015. Front Behav Neurosci. 2015. PMID: 26582981 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

