Biology of the RANKL-RANK-OPG System in Immunity, Bone, and Beyond
- PMID: 25368616
- PMCID: PMC4202272
- DOI: 10.3389/fimmu.2014.00511
Biology of the RANKL-RANK-OPG System in Immunity, Bone, and Beyond
Abstract
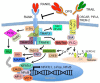
Discovery and characterization of the cytokine receptor-cytokine-decoy receptor triad formed by receptor activator of nuclear factor kappa-B ligand (RANKL)-receptor activator of NF-κB (RANK)-osteoprotegerin (OPG) have led not only to immense advances in understanding the biology of bone homeostasis, but have also crystalized appreciation of the critical regulatory relationship that exists between bone and immunity, resulting in the emergence of the burgeoning field of osteoimmunology. RANKL-RANK-OPG are members of the tumor necrosis factor (TNF) and TNF receptor superfamilies, and share signaling characteristics common to many members of each. Developmentally regulated and cell-type specific expression patterns of each of these factors have revealed key regulatory functions for RANKL-RANK-OPG in bone homeostasis, organogenesis, immune tolerance, and cancer. Successful efforts at designing and developing therapeutic agents targeting RANKL-RANK-OPG have been undertaken for osteoporosis, and additional efforts are underway for other conditions. In this review, we will summarize the basic biology of the RANKL-RANK-OPG system, relate its cell-type specific functions to system-wide mechanisms of development and homeostasis, and highlight emerging areas of interest for this cytokine group.
Keywords: RANKL; TNFRSF11; TNFSF11; TRAF6; TRANCE; mTECs; osteoimmunology; rheumatoid arthritis.
Figures

References
-
- Wong BR, Josien R, Lee SY, Sauter B, Li HL, Steinman RM, et al. TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell-specific survival factor. J Exp Med (1997) 186(12):2075–80.10.1084/jem.186.12.2075 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

