"Glowing head" mice: a genetic tool enabling reliable preclinical image-based evaluation of cancers in immunocompetent allografts
- PMID: 25369133
- PMCID: PMC4219677
- DOI: 10.1371/journal.pone.0109956
"Glowing head" mice: a genetic tool enabling reliable preclinical image-based evaluation of cancers in immunocompetent allografts
Abstract
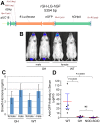
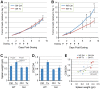
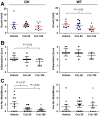
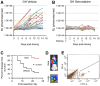
Preclinical therapeutic assessment currently relies on the growth response of established human cell lines xenografted into immunocompromised mice, a strategy that is generally not predictive of clinical outcomes. Immunocompetent genetically engineered mouse (GEM)-derived tumor allograft models offer highly tractable preclinical alternatives and facilitate analysis of clinically promising immunomodulatory agents. Imageable reporters are essential for accurately tracking tumor growth and response, particularly for metastases. Unfortunately, reporters such as luciferase and GFP are foreign antigens in immunocompetent mice, potentially hindering tumor growth and confounding therapeutic responses. Here we assessed the value of reporter-tolerized GEMs as allograft recipients by targeting minimal expression of a luciferase-GFP fusion reporter to the anterior pituitary gland (dubbed the "Glowing Head" or GH mouse). The luciferase-GFP reporter expressed in tumor cells induced adverse immune responses in wildtype mouse, but not in GH mouse, as transplantation hosts. The antigenicity of optical reporters resulted in a decrease in both the growth and metastatic potential of the labeled tumor in wildtype mice as compared to the GH mice. Moreover, reporter expression can also alter the tumor response to chemotherapy or targeted therapy in a context-dependent manner. Thus the GH mice and experimental approaches vetted herein provide concept validation and a strategy for effective, reproducible preclinical evaluation of growth and response kinetics for traceable tumors.
Conflict of interest statement
Figures


References
-
- Herper M (2012) The Truly Staggering Cost Of Inventing New Drugs. Forbes. Available: http://www.forbes.com/sites/matthewherper/2012/02/10/the-truly-staggerin....
-
- Sharpless NE, Depinho RA (2006) The mighty mouse: genetically engineered mouse models in cancer drug development. Nat Rev Drug Discov 5: 741–754. - PubMed
-
- Suggitt M, Bibby MC (2005) 50 years of preclinical anticancer drug screening: empirical to target-driven approaches. Clin Cancer Res 11: 971–981. - PubMed
-
- Administration USFaD (2004) Innovation or Stagnation: Challenge and Opportunity on the Critical Path to New Medical Products.
-
- Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G (2008) Immunological aspects of cancer chemotherapy. Nat Rev Immunol 8: 59–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

