Sublingual misoprostol versus intramuscular oxytocin for prevention of postpartum hemorrhage in Uganda: a double-blind randomized non-inferiority trial
- PMID: 25369200
- PMCID: PMC4219663
- DOI: 10.1371/journal.pmed.1001752
Sublingual misoprostol versus intramuscular oxytocin for prevention of postpartum hemorrhage in Uganda: a double-blind randomized non-inferiority trial
Abstract
Background: Postpartum hemorrhage (PPH) is a leading cause of maternal death in sub-Saharan Africa. Although the World Health Organization recommends use of oxytocin for prevention of PPH, misoprostol use is increasingly common owing to advantages in shelf life and potential for sublingual administration. There is a lack of data about the comparative efficacy of oxytocin and sublingual misoprostol, particularly at the recommended dose of 600 µg, for prevention of PPH during active management of labor.
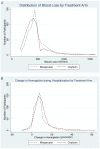
Methods and findings: We performed a double-blind, double-dummy randomized controlled non-inferiority trial between 23 September 2012 and 9 September 2013 at Mbarara Regional Referral Hospital in Uganda. We randomized 1,140 women to receive 600 µg of misoprostol sublingually or 10 IU of oxytocin intramuscularly, along with matching placebos for the treatment they did not receive. Our primary outcome of interest was PPH, defined as measured blood loss ≥ 500 ml within 24 h of delivery. Secondary outcomes included measured blood loss ≥ 1,000 ml; mean measured blood loss at 1, 2, and 24 h after delivery; death; requirement for blood transfusion; hemoglobin changes; and use of additional uterotonics. At 24 h postpartum, primary PPH occurred in 163 (28.6%) participants in the misoprostol group and 99 (17.4%) participants in the oxytocin group (relative risk [RR] 1.64, 95% CI 1.32 to 2.05, p<0.001; absolute risk difference 11.2%, 95% CI 6.44 to 16.1). Severe PPH occurred in 20 (3.6%) and 15 (2.7%) participants in the misoprostol and oxytocin groups, respectively (RR 1.33, 95% CI 0.69 to 2.58, p = 0.391; absolute risk difference 0.9%, 95% CI -1.12 to 2.88). Mean measured blood loss was 341.5 ml (standard deviation [SD] 206.2) and 304.2 ml (SD 190.8, p = 0.002) at 2 h and 484.7 ml (SD 213.3) and 432.8 ml (SD 203.5, p<0.001) at 24 h in the misoprostol and oxytocin groups, respectively. There were no significant differences between the two groups in any other secondary outcomes. Women in the misoprostol group more commonly experienced shivering (RR 1.91, 95% CI 1.65 to 2.21, p<0.001) and fevers (RR 5.20, 95% CI 3.15 to 7.21, p = 0.005). This study was conducted at a regional referral hospital with capacity for emergency surgery and blood transfusion. High-risk women were excluded from participation.
Conclusions: Misoprostol 600 µg is inferior to oxytocin 10 IU for prevention of primary PPH in active management of labor. These data support use of oxytocin in settings where it is available. While not powered to do so, the study found no significant differences in rate of severe PPH, need for blood transfusion, postpartum hemoglobin, change in hemoglobin, or use of additional uterotonics between study groups. Further research should focus on clarifying whether and in which sub-populations use of oxytocin would be preferred over sublingual misoprostol.
Trial registration: ClinicalTrials.gov NCT01866241 Please see later in the article for the Editors' Summary.
Conflict of interest statement
EAC, CO, MT, and AA previously worked with an independent EU-funded project aimed at documenting access to medicines in Africa and South Asia (AMASA) from where the gaps and research questions for this study were identified.
Figures
References
-
- World Health Organization, United Nations Children's Fund, United Nations Population Fund, World Bank (2012) Trends in maternal mortality: 1990–2010. WHO, UNICEF, UNFPA and The World Bank estimates. Geneva: World Health Organization.
-
- United Nations Department of Economic and Social Affairs Population Division (2013) World population prospects: the 2012 revision. New York: United Nations Department of Economic and Social Affairs Population Division.
-
- Uganda Ministry of Health (2010) Clinical guidelines for prevention and treatment of post partum haemorrhage using misoprostol. Kampala: Uganda Ministry of Health.
-
- World Health Organization (2012) WHO recommendations for the prevention and treatment of postpartum haemorrhage. Geneva: World Health Organization. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical