Effect of inorganic salts on the volatility of organic acids
- PMID: 25369247
- PMCID: PMC4255274
- DOI: 10.1021/es5033103
Effect of inorganic salts on the volatility of organic acids
Abstract
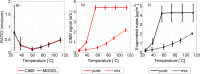
Particulate phase reactions between organic and inorganic compounds may significantly alter aerosol chemical properties, for example, by suppressing particle volatility. Here, chemical processing upon drying of aerosols comprised of organic (acetic, oxalic, succinic, or citric) acid/monovalent inorganic salt mixtures was assessed by measuring the evaporation of the organic acid molecules from the mixture using a novel approach combining a chemical ionization mass spectrometer coupled with a heated flow tube inlet (TPD-CIMS) with kinetic model calculations. For reference, the volatility, i.e. saturation vapor pressure and vaporization enthalpy, of the pure succinic and oxalic acids was also determined and found to be in agreement with previous literature. Comparison between the kinetic model and experimental data suggests significant particle phase processing forming low-volatility material such as organic salts. The results were similar for both ammonium sulfate and sodium chloride mixtures, and relatively more processing was observed with low initial aerosol organic molar fractions. The magnitude of low-volatility organic material formation at an atmospherically relevant pH range indicates that the observed phenomenon is not only significant in laboratory conditions but is also of direct atmospheric relevance.
Figures



References
-
- McFiggans G.; Artaxo P.; Baltensperger U.; Coe H.; Facchini M. C.; Feingold G.; Fuzzi S.; Gysel M.; Laaksonen A.; Lohmann U.; Mentel T. F.; Murphy D. M.; O’Dowd C. D.; Snider J. R.; Weingartner E. The effect of physical and chemical aerosol properties on warm cloud droplet activation. Atmos. Chem. Phys. 2006, 6, 2593–2649.
-
- Rosenfeld D.; Lohmann U.; Raga G. B.; O’Dowd C. D.; Kulmala M.; Fuzzi S.; Reissell A.; Andreae M. O. Flood or drought: how do aerosols affect precipitation?. Science 2008, 321, 1309–1313. - PubMed
-
- Jimenez J. L.; Canagaratna M. R.; Donahue N. M.; Prevot A. S.; Zhang Q.; Kroll J. H.; DeCarlo P. F.; Allan J. D.; Coe H.; Ng N. L.; Aiken A. C.; Docherty K. S.; Ulbrich I. M.; Grieshop A. P.; Robinson A. L.; Duplissy J.; Smith J. D.; Wilson K. R.; Lanz V. A.; Hueglin C.; Sun Y. L.; Tian J.; Laaksonen A.; Raatikainen T.; Rautiainen J.; Vaattovaara P.; Ehn M.; Kulmala M.; Tomlinson J. M.; Collins D. R.; Cubison M. J.; Dunlea E. J.; Huffman J. A.; Onasch T. B.; Alfarra M. R.; Williams P. I.; Bower K.; Kondo Y.; Schneider J.; Drewnick F.; Borrmann S.; Weimer S.; Demerjian K.; Salcedo D.; Cottrell L.; Griffin R.; Takami A.; Miyoshi T.; Hatakeyama S.; Shimono A.; Sun J. Y.; Zhang Y. M.; Dzepina K.; Kimmel J. R.; Sueper D.; Jayne J. T.; Herndon S. C.; Trimborn A. M.; Williams L. R.; Wood E. C.; Middlebrook A. M.; Kolb C. E.; Baltensperger U.; Worsnop D. R. Evolution of organic aerosols in the atmosphere. Science 2009, 326, 1525–1529. - PubMed
-
- Robinson A. L.; Donahue N. M.; Shrivastava M. K.; Weitkamp E. A.; Sage A. M.; Grieshop A. P.; Lane T. E.; Pierce J. R.; Pandis S. N. Rethinking organic aerosols: semivolatile emissions and photochemical aging. Science 2007, 315, 1259–1262. - PubMed
-
- Riipinen I.; Pierce J. R.; Yli-Juuti T.; Nieminen T.; Häkkinen S.; Ehn M.; Junninen H.; Lehtipalo K.; Petäjä T.; Slowik J.; Chang R.; Shantz N. C.; Abbatt J.; Leaitch W. R.; Kerminen V. M.; Worsnop D. R.; Pandis S. N.; Donahue N. M.; Kulmala M. Organic condensation: a vital link connecting aerosol formation to cloud condensation nuclei (CCN) concentrations. Atmos. Chem. Phys. 2011, 11, 3865–3878.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

