Criteria for viability assessment of discarded human donor livers during ex vivo normothermic machine perfusion
- PMID: 25369327
- PMCID: PMC4219693
- DOI: 10.1371/journal.pone.0110642
Criteria for viability assessment of discarded human donor livers during ex vivo normothermic machine perfusion
Abstract
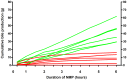
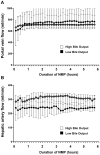
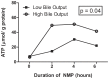
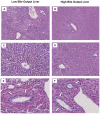
Although normothermic machine perfusion of donor livers may allow assessment of graft viability prior to transplantation, there are currently no data on what would be a good parameter of graft viability. To determine whether bile production is a suitable biomarker that can be used to discriminate viable from non-viable livers we have studied functional performance as well as biochemical and histological evidence of hepatobiliary injury during ex vivo normothermic machine perfusion of human donor livers. After a median duration of cold storage of 6.5 h, twelve extended criteria human donor livers that were declined for transplantation were ex vivo perfused for 6 h at 37 °C with an oxygenated solution based on red blood cells and plasma, using pressure controlled pulsatile perfusion of the hepatic artery and continuous portal perfusion. During perfusion, two patterns of bile flow were identified: (1) steadily increasing bile production, resulting in a cumulative output of ≥ 30 g after 6 h (high bile output group), and (2) a cumulative bile production <20 g in 6 h (low bile output group). Concentrations of transaminases and potassium in the perfusion fluid were significantly higher in the low bile output group, compared to the high bile output group. Biliary concentrations of bilirubin and bicarbonate were respectively 4 times and 2 times higher in the high bile output group. Livers in the low bile output group displayed more signs of hepatic necrosis and venous congestion, compared to the high bile output group. In conclusion, bile production could be an easily assessable biomarker of hepatic viability during ex vivo machine perfusion of human donor livers. It could potentially be used to identify extended criteria livers that are suitable for transplantation. These ex vivo findings need to be confirmed in a transplant experiment or a clinical trial.
Conflict of interest statement
Figures
References
-
- Merion RM, Goodrich NP, Feng S (2006) How can we define expanded criteria for liver donors? J Hepatol 45: 484–488. - PubMed
-
- McCormack L, Dutkowski P, El-Badry AM, Clavien PA (2011) Liver transplantation using fatty livers: always feasible? J Hepatol 54: 1055–1062. - PubMed
-
- Op den Dries S, Sutton ME, Lisman T, Porte RJ (2011) Protection of bile ducts in liver transplantation: looking beyond ischemia. Transplantation 92: 373–379. - PubMed
-
- Bae C, Henry SD, Guarrera JV (2012) Is extracorporeal hypothermic machine perfusion of the liver better than the 'good old icebox'? Curr Opin Organ Transplant 17: 137–142. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

