Hyperpolarized magnetic resonance as a sensitive detector of metabolic function
- PMID: 25369537
- PMCID: PMC4255644
- DOI: 10.1021/bi501225t
Hyperpolarized magnetic resonance as a sensitive detector of metabolic function
Abstract
Hyperpolarized magnetic resonance allows for noninvasive measurements of biochemical reactions in vivo. Although this technique provides a unique tool for assaying enzymatic activities in intact organs, the scope of its application is still elusive for the wider scientific community. The purpose of this review is to provide key principles and parameters to guide the researcher interested in adopting this technology to address a biochemical, biomedical, or medical issue. It is presented in the form of a compendium containing the underlying essential physical concepts as well as suggestions to help assess the potential of the technique within the framework of specific research environments. Explicit examples are used to illustrate the power as well as the limitations of hyperpolarized magnetic resonance.
Figures






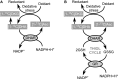

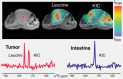
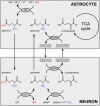
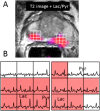
References
-
- Gruetter R.; Novotny E. J.; Boulware S. D.; Mason G. F.; Rothman D. L.; Shulman G. I.; Prichard J. W.; Shulman R. G. (1994) Localized C-13 Nmr-Spectroscopy in the Human Brain of Amino-Acid Labeling from d-[1-C-13]Glucose. J. Neurochem. 63, 1377–1385. - PubMed
-
- Gruetter R.; Seaquist E. R.; Kim S.; Ugurbil K. (1998) Localized in vivo C-13-NMR of glutamate metabolism in the human brain: Initial results at 4 T. Dev. Neurosci. (Basel, Switz.) 20, 380–388. - PubMed
-
- Maher E. A.; Marin-Valencia I.; Bachoo R. M.; Mashimo T.; Raisanen J.; Hatanpaa K. J.; Jindal A.; Jeffrey F. M.; Choi C. H.; Madden C.; Mathews D.; Pascual J. M.; Mickey B. E.; Malloy C. R.; DeBerardinis R. J. (2012) Metabolism of [U-13C]glucose in human brain tumors in vivo. NMR Biomed. 25, 1234–1244. - PMC - PubMed
-
- Shulman R. G.; Rothman D. L. (2001) 13C NMR of intermediary metabolism: Implications for systemic physiology. Annu. Rev. Physiol. 63, 15–48. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

