Endocannabinoids and striatal function: implications for addiction-related behaviours
- PMID: 25369747
- PMCID: PMC5398317
- DOI: 10.1097/FBP.0000000000000109
Endocannabinoids and striatal function: implications for addiction-related behaviours
Abstract
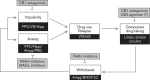
Since the identification and cloning of the major cannabinoid receptor expressed in the brain almost 25 years ago research has highlighted the potential of drugs that target the endocannabinoid system for treating addiction. The endocannabinoids, anandamide and 2-arachidonoyl glycerol, are lipid-derived metabolites found in abundance in the basal ganglia and other brain areas innervated by the mesocorticolimbic dopamine systems. Cannabinoid CB1 receptor antagonists/inverse agonists reduce reinstatement of responding for cocaine, alcohol and opiates in rodents. However, compounds acting on the endocannabinoid system may have broader application in treating drug addiction by ameliorating associated traits and symptoms such as impulsivity and anxiety that perpetuate drug use and interfere with rehabilitation. As a trait, impulsivity is known to predispose to addiction and facilitate the emergence of addiction to stimulant drugs. In contrast, anxiety and elevated stress responses accompany extended drug use and may underlie the persistence of drug intake in dependent individuals. In this article we integrate and discuss recent findings in rodents showing selective pharmacological modulation of impulsivity and anxiety by cannabinoid agents. We highlight the potential of selective inhibitors of endocannabinoid metabolism, directed at fatty acid amide hydrolase and monoacylglycerol lipase, to reduce anxiety and stress responses, and discuss novel mechanisms underlying the modulation of the endocannabinoid system, including the attenuation of impulsivity, anxiety, and drug reward by selective CB2 receptor agonists.
Figures


References
-
- Adams JB, Heath AJ, Young SE, Hewitt JK, Corley RP, Stallings MC. (2003). Relationships between personality and preferred substance and motivations for use among adolescent substance abusers. Am J Drug Alcohol Abuse 29:691–712. - PubMed
-
- Adriani W, Caprioli A, Granstrem O, Carli M, Laviola G. (2003). The spontaneously hypertensive-rat as an animal model of ADHD: evidence for impulsive and non-impulsive subpopulations. Neurosci Biobehav Rev 27:639–651. - PubMed
-
- Ansquer S, Belin-Rauscent A, Dugast E, Duran T, Benatru I, Mar AC, et al. (2014). Atomoxetine decreases vulnerability to develop compulsivity in high impulsive rats. Biol Psychiatry 75:825–832. - PubMed
-
- Arguello PA, Jentsch JD. (2004). Cannabinoid CB1 receptor-mediated impairment of visuospatial attention in the rat. Psychopharmacology (Berl) 177:141–150. - PubMed
-
- Arnone M, Maruani J, Chaperon F, Thiébot MH, Poncelet M, Soubrié P, Le Fur G. (1997). Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 132:104–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

