Efficacy of an Internet and SMS-based integrated smoking cessation and alcohol intervention for smoking cessation in young people: study protocol of a two-arm cluster randomised controlled trial
- PMID: 25369857
- PMCID: PMC4228117
- DOI: 10.1186/1471-2458-14-1140
Efficacy of an Internet and SMS-based integrated smoking cessation and alcohol intervention for smoking cessation in young people: study protocol of a two-arm cluster randomised controlled trial
Abstract
Background: Tobacco smoking prevalence continues to be high, particularly among adolescents and young adults with lower educational levels, and is therefore a serious public health problem. Tobacco smoking and problem drinking often co-occur and relapses after successful smoking cessation are often associated with alcohol use. This study aims at testing the efficacy of an integrated smoking cessation and alcohol intervention by comparing it to a smoking cessation only intervention for young people, delivered via the Internet and mobile phone.
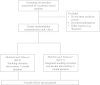
Methods/design: A two-arm cluster-randomised controlled trial with one follow-up assessment after 6 months will be conducted. Participants in the integrated intervention group will: (1) receive individually tailored web-based feedback on their drinking behaviour based on age and gender norms, (2) receive individually tailored mobile phone text messages to promote drinking within low-risk limits over a 3-month period, (3) receive individually tailored mobile phone text messages to support smoking cessation for 3 months, and (4) be offered the option of registering for a more intensive program that provides strategies for smoking cessation centred around a self-defined quit date. Participants in the smoking cessation only intervention group will only receive components (3) and (4). Study participants will be 1350 students who smoke tobacco daily/occasionally, from vocational schools in Switzerland. Main outcome criteria are 7-day point prevalence smoking abstinence and cigarette consumption assessed at the 6-month follow up.
Discussion: This is the first study testing a fully automated intervention for smoking cessation that simultaneously addresses alcohol use and interrelations between tobacco and alcohol use. The integrated intervention can be easily implemented in various settings and could be used with large groups of young people in a cost-effective way.
Trial registration: Current Controlled Trials ISRCTN02427446 (date of registration: 08th September, 2014).
References
-
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224–2260. doi: 10.1016/S0140-6736(12)61766-8. - DOI - PMC - PubMed
-
- Hibell B, Guttormsson U, Ahlström S, Balakireva O, Bjarnason T, Kokkevi A, Kraus L: The 2011 ESPAD Report. Substance Use among Students in 36 European Countries. Stockholm: The Swedish Council for Information on Alcohol and other Drugs;
-
- Stanton A, Grimshaw G. Tobacco cessation interventions for young people. Cochrane Database Syst Rev. 2013;8:CD003289. - PubMed
-
- Willemse I, Waller G, Süss D, Genner S, Huber A-L. JAMES: Jugend, Aktivitäten, Medien - Erhebung Schweiz [JAMES: Youth, Activity, Media - Data Switzerland] Zürich: Zürcher Hochschule für angewandte Wissenschaften; 2012.
Pre-publication history
-
- The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2458/14/1140/prepub
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical