Complete and ubiquitinated proteome of the Legionella-containing vacuole within human macrophages
- PMID: 25369898
- PMCID: PMC4286187
- DOI: 10.1021/pr500765x
Complete and ubiquitinated proteome of the Legionella-containing vacuole within human macrophages
Abstract
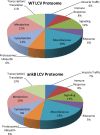
Within protozoa or human macrophages Legionella pneumophila evades the endosomal pathway and replicates within an ER-derived vacuole termed the Legionella-containing vacuole (LCV). The LCV membrane-localized AnkB effector of L. pneumophila is an F-box protein that mediates decoration of the LCV with lysine(48)-linked polyubiquitinated proteins, which is essential for intravacuolar replication. Using high-throughput LC-MS analysis, we have identified the total and ubiquitinated host-derived proteome of LCVs purified from human U937 macrophages. The LCVs harboring the AA100/130b WT strain contain 1193 proteins including 24 ubiquitinated proteins, while the ankB mutant LCVs contain 1546 proteins with 29 ubiquitinated proteins. Pathway analyses reveal the enrichment of proteins involved in signaling, protein transport, phosphatidylinositol, and carbohydrate metabolism on both WT and ankB mutant LCVs. The ankB mutant LCVs are preferentially enriched for proteins involved in transcription/translation and immune responses. Ubiquitinated proteins on the WT strain LCVs are enriched for immune response, signaling, regulation, intracellular trafficking, and amino acid transport pathways, while ubiquitinated proteins on the ankB mutant LCVs are enriched for vesicle trafficking, signaling, and ubiquitination pathways. The complete and ubiquitinated LCV proteome within human macrophages illustrates complex and dynamic biogenesis of the LCV and provides a rich resource for future studies.
Keywords: AnkB; Dot/Icm; LCV; Legionnaires’ disease; polyubiquitin; proteome; signaling.
Figures

Similar articles
-
Comparative Proteomics of Purified Pathogen Vacuoles Correlates Intracellular Replication of Legionella pneumophila with the Small GTPase Ras-related protein 1 (Rap1).Mol Cell Proteomics. 2017 Apr;16(4):622-641. doi: 10.1074/mcp.M116.063453. Epub 2017 Feb 9. Mol Cell Proteomics. 2017. PMID: 28183814 Free PMC article.
-
Molecular mimicry by an F-box effector of Legionella pneumophila hijacks a conserved polyubiquitination machinery within macrophages and protozoa.PLoS Pathog. 2009 Dec;5(12):e1000704. doi: 10.1371/journal.ppat.1000704. Epub 2009 Dec 24. PLoS Pathog. 2009. PMID: 20041211 Free PMC article.
-
Legionella-Containing Vacuoles Capture PtdIns(4)P-Rich Vesicles Derived from the Golgi Apparatus.mBio. 2018 Dec 11;9(6):e02420-18. doi: 10.1128/mBio.02420-18. mBio. 2018. PMID: 30538188 Free PMC article.
-
Autophagy Evasion and Endoplasmic Reticulum Subversion: The Yin and Yang of Legionella Intracellular Infection.Annu Rev Microbiol. 2016 Sep 8;70:413-33. doi: 10.1146/annurev-micro-102215-095557. Annu Rev Microbiol. 2016. PMID: 27607556 Review.
-
Phosphoinositides and the Fate of Legionella in Phagocytes.Front Immunol. 2020 Jan 30;11:25. doi: 10.3389/fimmu.2020.00025. eCollection 2020. Front Immunol. 2020. PMID: 32117224 Free PMC article. Review.
Cited by
-
Type II Secretion Is Necessary for Optimal Association of the Legionella-Containing Vacuole with Macrophage Rab1B but Enhances Intracellular Replication Mainly by Rab1B-Independent Mechanisms.Infect Immun. 2016 Nov 18;84(12):3313-3327. doi: 10.1128/IAI.00750-16. Print 2016 Dec. Infect Immun. 2016. PMID: 27600508 Free PMC article.
-
Nutrition and Bipartite Metabolism of Intracellular Pathogens.Trends Microbiol. 2019 Jun;27(6):550-561. doi: 10.1016/j.tim.2018.12.012. Epub 2019 Jan 14. Trends Microbiol. 2019. PMID: 30655036 Free PMC article. Review.
-
Protein and DNA Biosynthesis Demonstrated in Host Cell-Free Phagosomes Containing Anaplasma phagocytophilum or Ehrlichia chaffeensis in Axenic Media.Infect Immun. 2021 Mar 17;89(4):e00638-20. doi: 10.1128/IAI.00638-20. Print 2021 Mar 17. Infect Immun. 2021. PMID: 33431703 Free PMC article.
-
A MicroRNA Network Controls Legionella pneumophila Replication in Human Macrophages via LGALS8 and MX1.mBio. 2020 Mar 24;11(2):e03155-19. doi: 10.1128/mBio.03155-19. mBio. 2020. PMID: 32209695 Free PMC article.
-
Isolation of F. novicida-Containing Phagosome from Infected Human Monocyte Derived Macrophages.Front Cell Infect Microbiol. 2017 Jul 5;7:303. doi: 10.3389/fcimb.2017.00303. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28725638 Free PMC article.
References
-
- Kagan J. C.; Roy C. R. Legionella phagosomes intercept vesicular traffic from endoplasmic reticulum exit sites. Nat. Cell Biol. 2002, 412945–954. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

