Cerebral small vessel disease-related protease HtrA1 processes latent TGF-β binding protein 1 and facilitates TGF-β signaling
- PMID: 25369932
- PMCID: PMC4246310
- DOI: 10.1073/pnas.1418087111
Cerebral small vessel disease-related protease HtrA1 processes latent TGF-β binding protein 1 and facilitates TGF-β signaling
Abstract
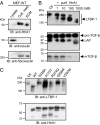
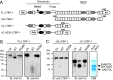
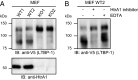
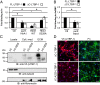
High temperature requirement protein A1 (HtrA1) is a primarily secreted serine protease involved in a variety of cellular processes including transforming growth factor β (TGF-β) signaling. Loss of its activity causes cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy (CARASIL), an inherited form of cerebral small vessel disease leading to early-onset stroke and premature dementia. Dysregulated TGF-β signaling is considered to promote CARASIL pathogenesis, but the underlying molecular mechanisms are incompletely understood. Here we present evidence from mouse brain tissue and embryonic fibroblasts as well as patient skin fibroblasts for a facilitating role of HtrA1 in TGF-β pathway activation. We identify latent TGF-β binding protein 1 (LTBP-1), an extracellular matrix protein and key regulator of TGF-β bioavailability, as a novel HtrA1 target. Cleavage occurs at physiological protease concentrations, is prevented under HtrA1-deficient conditions as well as by CARASIL mutations and disrupts both LTBP-1 binding to fibronectin and its incorporation into the extracellular matrix. Hence, our data suggest an attenuation of TGF-β signaling caused by a lack of HtrA1-mediated LTBP-1 processing as mechanism underlying CARASIL pathogenesis.
Keywords: LTBP-1; extracellular matrix; proteolysis; small vessel disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Reply to Liu et al.: Loss of TGF-β signaling in CARASIL pathogenesis.Proc Natl Acad Sci U S A. 2015 Apr 7;112(14):E1694. doi: 10.1073/pnas.1501817112. Epub 2015 Mar 13. Proc Natl Acad Sci U S A. 2015. PMID: 25770223 Free PMC article. No abstract available.
-
Loss of HtrA1-induced attenuation of TGF-β signaling in fibroblasts might not be the main mechanism of CARASIL pathogenesis.Proc Natl Acad Sci U S A. 2015 Apr 7;112(14):E1693. doi: 10.1073/pnas.1500911112. Epub 2015 Mar 13. Proc Natl Acad Sci U S A. 2015. PMID: 25770224 Free PMC article. No abstract available.
References
-
- Dichgans M. Genetics of ischaemic stroke. Lancet Neurol. 2007;6(2):149–161. - PubMed
-
- Federico A, et al. Hereditary cerebral small vessel diseases: A review. J Neurol Sci. 2012;322(1-2):25–30. - PubMed
-
- Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser MG. Cadasil. Lancet Neurol. 2009;8(7):643–653. - PubMed
-
- Fukutake T. Cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy (CARASIL): From discovery to gene identification. J Stroke Cerebrovasc Dis. 2011;20(2):85–93. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

