Maresin 1 biosynthesis during platelet-neutrophil interactions is organ-protective
- PMID: 25369934
- PMCID: PMC4246348
- DOI: 10.1073/pnas.1407123111
Maresin 1 biosynthesis during platelet-neutrophil interactions is organ-protective
Abstract
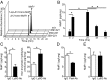
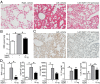
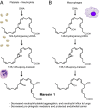
Unregulated acute inflammation can lead to collateral tissue injury in vital organs, such as the lung during the acute respiratory distress syndrome. In response to tissue injury, circulating platelet-neutrophil aggregates form to augment neutrophil tissue entry. These early cellular events in acute inflammation are pivotal to timely resolution by mechanisms that remain to be elucidated. Here, we identified a previously undescribed biosynthetic route during human platelet-neutrophil interactions for the proresolving mediator maresin 1 (MaR1; 7R,14S-dihydroxy-docosa-4Z,8E,10E,12Z,16Z,19Z-hexaenoic acid). Docosahexaenoic acid was converted by platelet 12-lipoxygenase to 13S,14S-epoxy-maresin, which was further transformed by neutrophils to MaR1. In a murine model of acute respiratory distress syndrome, lipid mediator metabololipidomics uncovered MaR1 generation in vivo in a temporally regulated manner. Early MaR1 production was dependent on platelet-neutrophil interactions, and intravascular MaR1 was organ-protective, leading to decreased lung neutrophils, edema, tissue hypoxia, and prophlogistic mediators. Together, these findings identify a transcellular route for intravascular maresin 1 biosynthesis via platelet-neutrophil interactions that regulates the extent of lung inflammation.
Keywords: inflammation; lung; maresin; platelet; resolution.
Conflict of interest statement
Conflict of interest statement: C.N.S. is an inventor on patents (resolvins) assigned to Brigham and Women's Hospital (BWH) and licensed to Resolvyx Pharmaceuticals. C.N.S. was the scientific founder of Resolvyx Pharmaceuticals and owns founder stock in the company. C.N.S.’s interests were reviewed and are managed by the Brigham and Women's Hospital and Partners HealthCare in accordance with their conflict of interest policies. B.D.L. is an inventor on patents (resolvins) assigned to BWH and licensed to Resolvyx Pharmaceuticals. B.D.L.’s interests were reviewed and are managed by the Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies.
Figures


References
-
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7(9):678–689. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

