Incorporation of a prolyl hydroxylase inhibitor into scaffolds: a strategy for stimulating vascularization
- PMID: 25370818
- PMCID: PMC4356217
- DOI: 10.1089/ten.TEA.2014.0077
Incorporation of a prolyl hydroxylase inhibitor into scaffolds: a strategy for stimulating vascularization
Abstract
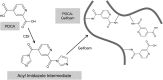
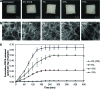
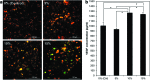
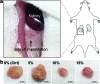
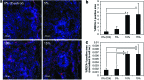
Clinical applications of tissue engineering are constrained by the ability of the implanted construct to invoke vascularization in adequate extent and velocity. To overcome the current limitations presented by local delivery of single angiogenic factors, we explored the incorporation of prolyl hydroxylase inhibitors (PHIs) into scaffolds as an alternative vascularization strategy. PHIs are small molecule drugs that can stabilize the alpha subunit of hypoxia-inducible factor-1 (HIF-1), a key transcription factor that regulates a variety of angiogenic mechanisms. In this study, we conjugated the PHI pyridine-2,4-dicarboxylic acid (PDCA) through amide bonds to a gelatin sponge (Gelfoam(®)). Fibroblasts cultured on PDCA-Gelfoam were able to infiltrate and proliferate in these scaffolds while secreting significantly more vascular endothelial growth factor than cells grown on Gelfoam without PDCA. Reporter cells expressing green fluorescent protein-tagged HIF-1α exhibited dose-dependent stabilization of this angiogenic transcription factor when growing within PDCA-Gelfoam constructs. Subsequently, we implanted PDCA-Gelfoam scaffolds into the perirenal fat tissue of Sprague Dawley rats for 8 days. Immunostaining of explants revealed that the PDCA-Gelfoam scaffolds were amply infiltrated by cells and promoted vascular ingrowth in a dose-dependent manner. Thus, the incorporation of PHIs into scaffolds appears to be a feasible strategy for improving vascularization in regenerative medicine applications.
Figures


References
-
- Johnson P.C., Mikos A.G., Fisher J.P., and Jansen J.A.Strategic directions in tissue engineering. Tissue Eng 13,2827, 2007 - PubMed
-
- Novosel E.C., Kleinhans C., and Kluger P.J.Vascularization is the key challenge in tissue engineering. Adv Drug Deliv Rev 63,300, 2011 - PubMed
-
- Naderi H., Matin M.M., and Bahrami A.R.Review paper: critical issues in tissue engineering: biomaterials, cell sources, angiogenesis, and drug delivery systems. J Biomater Appl 26,383, 2011 - PubMed
-
- Rouwkema J., Rivron N.C., and van Blitterswijk C.A.Vascularization in tissue engineering. Trends Biotechnol 26,434, 2008 - PubMed
-
- Carmeliet P., and Jain R.K.Angiogenesis in cancer and other diseases. Nature 407,249, 2000 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

