PRONOUNCE: randomized, open-label, phase III study of first-line pemetrexed + carboplatin followed by maintenance pemetrexed versus paclitaxel + carboplatin + bevacizumab followed by maintenance bevacizumab in patients ith advanced nonsquamous non-small-cell lung cancer
- PMID: 25371077
- PMCID: PMC4276572
- DOI: 10.1097/JTO.0000000000000366
PRONOUNCE: randomized, open-label, phase III study of first-line pemetrexed + carboplatin followed by maintenance pemetrexed versus paclitaxel + carboplatin + bevacizumab followed by maintenance bevacizumab in patients ith advanced nonsquamous non-small-cell lung cancer
Abstract
Introduction: PRONOUNCE compared the efficacy and safety of pemetrexed+carboplatin followed by pemetrexed (Pem+Cb) with paclitaxel+carboplatin+bevacizumab followed by bevacizumab (Pac+Cb+Bev) in patients with advanced nonsquamous non-small-cell lung cancer (NSCLC).
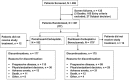
Methods: Patients ≥18 years of age with stage IV nonsquamous NSCLC (American Joint Committee on Cancer v7.0), and Eastern Cooperative Oncology Group performance status 0/1 were randomized (1:1) to four cycles of induction Pem+Cb (pemetrexed, 500 mg/m, carboplatin, area under the curve = 6) followed by Pem maintenance or Pac+Cb+Bev (paclitaxel, 200 mg/m, carboplatin, area under the curve = 6, and bevacizumab, 15 mg/kg) followed by Bev maintenance in the absence of progressive disease or discontinuation. The primary objective was progression-free survival (PFS) without grade 4 toxicity (G4PFS). Secondary end points were PFS, overall survival (OS), overall response rate (ORR), disease control rate (DCR), and safety. Resource utilization was also assessed.
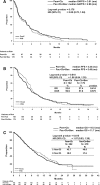
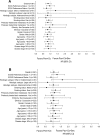
Results: Baseline characteristics of the patients randomized to Pem+Cb (N = 182) and Pac+Cb+Bev (N = 179) were well balanced between the arms. Median (months) G4PFS was 3.91 for Pem+Cb and 2.86 for Pac+Cb+Bev (hazard ratio = 0.85, 90% confidence interval, 0.7-1.04; p = 0.176); PFS, OS, ORR, or DCR did not differ significantly between the arms. Significantly more drug-related grade 3/4 anemia (18.7% versus 5.4%) and thrombocytopenia (24.0% versus 9.6%) were reported for Pem+Cb. Significantly more grade 3/4 neutropenia (48.8% versus 24.6%), grade 1/2 alopecia (28.3% versus 8.2%), and grade 1/2 sensory neuropathy were reported for Pac+Cb+Bev. Number of hospitalizations and overall length of stay did not differ significantly between the arms.
Conclusions: Pem+Cb did not produce significantly better G4PFS compared with Pac+Cb+Bev. Pem+Cb was not superior in PFS, OS, ORR, or DCR compared with Pac+Cb+Bev. Both regimens were well tolerated, although, toxicity profiles differed.
Trial registration: ClinicalTrials.gov NCT00948675.
Conflict of interest statement
Disclosure: Borys Hrinczenko, J. Thaddeus Beck, Manuel R. Modiano, Robert W. Weaver, David R. Spigel, and Helen J. Ross have no conflict of interest to declare. Ralph G. Zinner received funding for research from Eli Lilly and Company. Petros G. Nikolinakos participated in the advisory boards meetings and received honoraria from Eli Lilly and Company. David M. Waterhouse was on the speaker’s bureau for Eli Lilly and Company and received honoraria in the past. Ramaswamy Govindan is a consultant for Boehringer Ingelheim, GlaxoSmithKline, Pfizer, Merck, Bayer, Covidien, Bristol-Myers Squibb, Genentech, and Mallinckrodt. Coleman K. Obasaju, Jingyi Liu, Andrew G. Koustenis, Katherine B. Winfree, Symantha A. Melemed, Susan C. Guba, Waldo I. Ortuzar, Durisala Desaiah, and Joseph A. Treat are employees of Eli Lilly and Company and may hold company stock.
Figures
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. - PubMed
-
- Obasaju CK, Raju RN, Stinchcombe T, et al. Final results of a randomized phase II trial of pemetrexed (P) + carboplatin (Cb) ± enzastaurin (E) versus docetaxel (D) + Cb as first-line treatment of patients (pts) with stage IIIB/IV non–small-cell lung cancer (NSCLC). J Clin Oncol. 2009;27(suppl abstract 8037):15s.
-
- Grilli R, Oxman AD, Julian JA. Chemotherapy for advanced non-small-cell lung cancer: how much benefit is enough? J Clin Oncol. 1993;11:1866–1872. - PubMed
-
- Marino P, Pampallona S, Preatoni A, Cantoni A, Invernizzi F. Chemotherapy vs supportive care in advanced non-small-cell lung cancer. Results of a meta-analysis of the literature. Chest. 1994;106:861–865. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

