Non-canonical interleukin 23 receptor complex assembly: p40 protein recruits interleukin 12 receptor β1 via site II and induces p19/interleukin 23 receptor interaction via site III
- PMID: 25371211
- PMCID: PMC4281739
- DOI: 10.1074/jbc.M114.617597
Non-canonical interleukin 23 receptor complex assembly: p40 protein recruits interleukin 12 receptor β1 via site II and induces p19/interleukin 23 receptor interaction via site III
Abstract
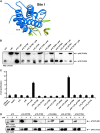
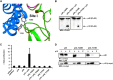
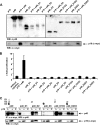
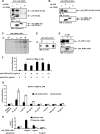
IL-23, composed of the cytokine subunit p19 and the soluble α receptor subunit p40, binds to a receptor complex consisting of the IL-23 receptor (IL-23R) and the IL-12 receptor β1 (IL-12Rβ1). Complex formation was hypothesized to follow the "site I-II-III" architectural paradigm, with site I of p19 being required for binding to p40, whereas sites II and III of p19 mediate binding to IL-12Rβ1 and IL-23R, respectively. Here we show that the binding mode of p19 to p40 and of p19 to IL-23R follow the canonical site I and III paradigm but that interaction of IL-23 to IL-12Rβ1 is independent of site II in p19. Instead, binding of IL-23 to the cytokine binding module of IL-12Rβ1 is mediated by domains 1 and 2 of p40 via corresponding site II amino acids of IL-12Rβ1. Moreover, domains 2 and 3 of p40 were sufficient for complex formation with p19 and to induce binding of p19 to IL-23R. The Fc-tagged fusion protein of p40_D2D3/p19 did, however, not act as a competitive IL-23 antagonist but, at higher concentrations, induced proliferation via IL-23R but independent of IL-12Rβ1. On the basis of our experimental validation, we propose a non-canonical topology of the IL-23·IL-23R·IL-12Rβ1 complex. Furthermore, our data help to explain why p40 is an antagonist of IL-23 and IL-12 signaling and show that site II of p19 is dispensable for IL-23 signaling.
Keywords: Cytokine; Cytokine Action; Interleukin; Interleukin 12 Receptor; Interleukin 23; Interleukin 23 Receptor; Molecular Modeling; Mutagenesis; p19; p40.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Garbers C., Hermanns H. M., Schaper F., Müller-Newen G., Grötzinger J., Rose-John S., Scheller J. (2012) Plasticity and cross-talk of interleukin 6-type cytokines. Cytokine Growth Factor Rev. 23, 85–97 - PubMed
-
- Korn T., Bettelli E., Oukka M., Kuchroo V. K. (2009) IL-17 and Th17 Cells. Annu. Rev. Immunol. 27, 485–517 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

