Kinetics of iron import into developing mouse organs determined by a pup-swapping method
- PMID: 25371212
- PMCID: PMC4281753
- DOI: 10.1074/jbc.M114.606731
Kinetics of iron import into developing mouse organs determined by a pup-swapping method
Abstract
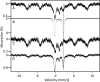
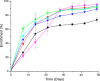
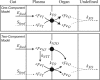
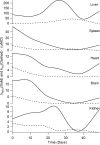
The kinetics of dietary iron import into various organs of mice were evaluated using a novel pup-swapping approach. Newborn pups whose bodies primarily contained (56)Fe or (57)Fe were swapped at birth such that each nursed on milk containing the opposite isotope. A pup from each litter was euthanized weekly over a 7-week period. Blood plasma was obtained, and organs were isolated typically after flushing with Ringer's buffer. (56)Fe and (57)Fe concentrations were determined for organs and plasma; organ volumes were also determined. Mössbauer spectra of equivalent (57)Fe-enriched samples were used to quantify residual blood in organs; this fraction was excluded from later analysis. Rates of import into brain, spleen, heart, and kidneys were highest during the first 2 weeks of life. In contrast, half of iron in the newborn liver exited during that time, and influx peaked later. Two mathematical models were developed to analyze the import kinetics. The only model that simulated the data adequately assumed that an iron-containing species enters the plasma and converts into a second species and that both are independently imported into organs. Consistent with this, liquid chromatography with an on-line ICP-MS detector revealed numerous iron species in plasma besides transferrin. Model fitting required that the first species, assigned to non-transferrin-bound iron, imports faster into organs than the second, assigned to transferrin-bound-iron. Non-transferrin-bound iron rather than transferrin-bound-iron appears to play the dominant role in importing iron into organs during early development of healthy mice.
Keywords: Iron Metabolism; Mathematical Modeling; Mossbauer Spectroscopy; Plasma; Transferrin.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Ganz T. (2013) Systemic iron homeostasis. Physiol. Rev. 93, 1721–1741 - PubMed
-
- Erikson K. M., Pinero D. J., Connor J. R., Beard J. L. (1997) Regional brain iron, ferritin and transferrin concentrations during iron deficiency and iron repletion in developing rats. J. Nutr. 127, 2030–2038 - PubMed
-
- Siimes M. A., Refino C., Dallman P. R. (1980) Physiological anemia of early development in the rat – characterization of the iron-responsive component. Am. J. Clin. Nutr. 33, 2601–2608 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

