Periplasmic Binding Proteins in Thermophiles: Characterization and Potential Application of an Arginine-Binding Protein from Thermotoga maritima: A Brief Thermo-Story
- PMID: 25371336
- PMCID: PMC4187188
- DOI: 10.3390/life3010149
Periplasmic Binding Proteins in Thermophiles: Characterization and Potential Application of an Arginine-Binding Protein from Thermotoga maritima: A Brief Thermo-Story
Abstract
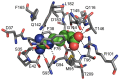
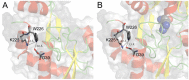
Arginine-binding protein from the extremophile Thermotoga maritima is a 27.7 kDa protein possessing the typical two-domain structure of the periplasmic binding proteins family. The protein is characterized by a very high specificity and affinity to bind to arginine, also at high temperatures. Due to its features, this protein could be taken into account as a potential candidate for the design of a biosensor for arginine. It is important to investigate the stability of proteins when they are used for biotechnological applications. In this article, we review the structural and functional features of an arginine-binding protein from the extremophile Thermotoga maritima with a particular eye on its potential biotechnological applications.
Figures


Similar articles
-
Amino acid transport in thermophiles: characterization of an arginine-binding protein from Thermotoga maritima. 3. Conformational dynamics and stability.J Photochem Photobiol B. 2013 Jan 5;118:66-73. doi: 10.1016/j.jphotobiol.2012.11.004. Epub 2012 Nov 29. J Photochem Photobiol B. 2013. PMID: 23232322
-
Amino acid transport in thermophiles: characterization of an arginine-binding protein in Thermotoga maritima.Mol Biosyst. 2010 Jan;6(1):142-51. doi: 10.1039/b908412f. Epub 2009 Sep 22. Mol Biosyst. 2010. PMID: 20024076
-
Amino acid transport in thermophiles: characterization of an arginine-binding protein in Thermotoga maritima. 2. Molecular organization and structural stability.Mol Biosyst. 2010 Apr;6(4):687-98. doi: 10.1039/b922092e. Epub 2010 Feb 22. Mol Biosyst. 2010. PMID: 20237647
-
Arginine regulation in Thermotoga neapolitana and Thermotoga maritima.Biochem Soc Trans. 2004 Apr;32(Pt 2):310-3. doi: 10.1042/bst0320310. Biochem Soc Trans. 2004. PMID: 15046597 Review.
-
Glyceraldehyde-3-phosphate dehydrogenase from Thermotoga maritima: strategies of protein stabilization.FEMS Microbiol Rev. 1996 May;18(2-3):215-24. doi: 10.1111/j.1574-6976.1996.tb00238.x. FEMS Microbiol Rev. 1996. PMID: 8639329 Review.
Cited by
-
Characterization of Cationic Amino Acid Binding Protein from Candidatus Liberibacter Asiaticus and in Silico Study to Identify Potential Inhibitor Molecules.Protein J. 2024 Oct;43(5):967-982. doi: 10.1007/s10930-024-10233-w. Epub 2024 Sep 21. Protein J. 2024. PMID: 39306651
-
Construction of a robust and sensitive arginine biosensor through ancestral protein reconstruction.Protein Sci. 2015 Sep;24(9):1412-22. doi: 10.1002/pro.2721. Epub 2015 Aug 18. Protein Sci. 2015. PMID: 26061224 Free PMC article.
-
A loose domain swapping organization confers a remarkable stability to the dimeric structure of the arginine binding protein from Thermotoga maritima.PLoS One. 2014 May 15;9(5):e96560. doi: 10.1371/journal.pone.0096560. eCollection 2014. PLoS One. 2014. PMID: 24832102 Free PMC article.
-
Development of a Protein Scaffold for Arginine Sensing Generated through the Dissection of the Arginine-Binding Protein from Thermotoga maritima.Int J Mol Sci. 2020 Oct 12;21(20):7503. doi: 10.3390/ijms21207503. Int J Mol Sci. 2020. PMID: 33053818 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

