Stem cells and the impact of ROS signaling
- PMID: 25371358
- PMCID: PMC4302918
- DOI: 10.1242/dev.107086
Stem cells and the impact of ROS signaling
Abstract
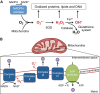
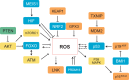
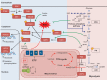
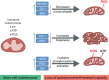
An appropriate balance between self-renewal and differentiation is crucial for stem cell function during both early development and tissue homeostasis throughout life. Recent evidence from both pluripotent embryonic and adult stem cell studies suggests that this balance is partly regulated by reactive oxygen species (ROS), which, in synchrony with metabolism, mediate the cellular redox state. In this Primer, we summarize what ROS are and how they are generated in the cell, as well as their downstream molecular targets. We then review recent findings that provide molecular insights into how ROS signaling can influence stem cell homeostasis and lineage commitment, and discuss the implications of this for reprogramming and stem cell ageing. We conclude that ROS signaling is an emerging key regulator of multiple stem cell populations.
Keywords: Embryonic stem cells; Hematopoietic stem cells; Metabolism; Mitochondria; ROS.
© 2014. Published by The Company of Biologists Ltd.
Figures
References
-
- Abbas H. A., MacCio D. R., Coskun S., Jackson J. G., Hazen A. L., Sills T. M., You M. J., Hirschi K. K. and Lozano G. (2010). Mdm2 is required for survival of hematopoietic stem cells/progenitors via dampening of ROS-induced p53 activity. Cell Stem Cell 7, 606-617 10.1016/j.stem.2010.09.013 - DOI - PMC - PubMed
-
- Anastasiou D., Poulogiannis G., Asara J. M., Boxer M. B., Jiang J.-k., Shen M., Bellinger G., Sasaki A. T., Locasale J. W., Auld D. S. et al. (2011). Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science 334, 1278-1283 10.1126/science.1211485 - DOI - PMC - PubMed
-
- Armstrong L., Tilgner K., Saretzki G., Atkinson S. P., Stojkovic M., Moreno R., Przyborski S. and Lako M. (2010). Human induced pluripotent stem cell lines show stress defense mechanisms and mitochondrial regulation similar to those of human embryonic stem cells. Stem Cells 28, 661-673 10.1002/stem.307 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

