First population-level effectiveness evaluation of a national programme to prevent HIV transmission from mother to child, South Africa
- PMID: 25371480
- PMCID: PMC4345523
- DOI: 10.1136/jech-2014-204535
First population-level effectiveness evaluation of a national programme to prevent HIV transmission from mother to child, South Africa
Abstract
Background: There is a paucity of data on the national population-level effectiveness of preventing mother-to-child transmission (PMTCT) programmes in high-HIV-prevalence, resource-limited settings. We assessed national PMTCT impact in South Africa (SA), 2010.
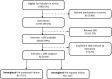
Methods: A facility-based survey was conducted using a stratified multistage, cluster sampling design. A nationally representative sample of 10 178 infants aged 4-8 weeks was recruited from 565 clinics. Data collection included caregiver interviews, record reviews and infant dried blood spots to identify HIV-exposed infants (HEI) and HIV-infected infants. During analysis, self-reported antiretroviral (ARV) use was categorised: 1a: triple ARV treatment; 1b: azidothymidine >10 weeks; 2a: azidothymidine ≤10 weeks; 2b: incomplete ARV prophylaxis; 3a: no antenatal ARV and 3b: missing ARV information. Findings were adjusted for non-response, survey design and weighted for live-birth distributions.
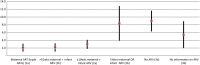
Results: Nationally, 32% of live infants were HEI; early mother-to-child transmission (MTCT) was 3.5% (95% CI 2.9% to 4.1%). In total 29.4% HEI were born to mothers on triple ARV treatment (category 1a) 55.6% on prophylaxis (1b, 2a, 2b), 9.5% received no antenatal ARV (3a) and 5.5% had missing ARV information (3b). Controlling for other factors groups, 1b and 2a had similar MTCT to 1a (Ref; adjusted OR (AOR) for 1b, 0.98, 0.52 to 1.83; and 2a, 1.31, 0.69 to 2.48). MTCT was higher in group 2b (AOR 3.68, 1.69 to 7.97). Within group 3a, early MTCT was highest among breastfeeding mothers 11.50% (4.67% to 18.33%) for exclusive breast feeding, 11.90% (7.45% to 16.35%) for mixed breast feeding, and 3.45% (0.53% to 6.35%) for no breast feeding). Antiretroviral therapy or >10 weeks prophylaxis negated this difference (MTCT 3.94%, 1.98% to 5.90%; 2.07%, 0.55% to 3.60% and 2.11%, 1.28% to 2.95%, respectively).
Conclusions: SA, a high-HIV-prevalence middle income country achieved <5% MTCT by 4-8 weeks post partum. The long-term impact on PMTCT on HIV-free survival needs urgent assessment.
Keywords: CHILD HEALTH; HIV; PERINATAL EPIDEMIOLOGY; PUBLIC HEALTH; SURVEILLANCE.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures

References
-
- United Nations. The Millennium Development Goal Report 2012. http://www.undp.org/content/dam/undp/library/MDG/english/The_MDG_Report_.... (accessed 8 Jul 2013).
-
- The Petra Study Team. Efficacy of three short-course regimens of zidovudine and lamivudine in preventing early and late transmission of HIV-1 from mother-to-child in Tanzania, South Africa and Uganda (Petra study): a randomised, double-blind plaebo-controlled trial. Lancet 1999;353:1178–86. - PubMed
-
- Lallemant M, Jourdain G, Le Coeur S, et al. . Single-dose perinatal nevirapine plus standard zidovudine to prevent mother-to-child transmission of HIV-1 in Thailand. N Engl J Med 2004;351:217–28. - PubMed
-
- Ayouba A, Tene G, Cunin P, et al. . Low rate of mother-to-child transmission of HIV-1 after nevirapine intervention in a public pilot health programme in Yaounde, Cameroon. J Acquir Immune Defic Syndr 2003;34:274–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
