Involvement of the electrophilic isothiocyanate sulforaphane in Arabidopsis local defense responses
- PMID: 25371552
- PMCID: PMC4281013
- DOI: 10.1104/pp.114.251892
Involvement of the electrophilic isothiocyanate sulforaphane in Arabidopsis local defense responses
Abstract
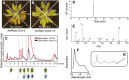
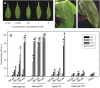
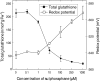
Plants defend themselves against microbial pathogens through a range of highly sophisticated and integrated molecular systems. Recognition of pathogen-secreted effector proteins often triggers the hypersensitive response (HR), a complex multicellular defense reaction where programmed cell death of cells surrounding the primary site of infection is a prominent feature. Even though the HR was described almost a century ago, cell-to-cell factors acting at the local level generating the full defense reaction have remained obscure. In this study, we sought to identify diffusible molecules produced during the HR that could induce cell death in naive tissue. We found that 4-methylsulfinylbutyl isothiocyanate (sulforaphane) is released by Arabidopsis (Arabidopsis thaliana) leaf tissue undergoing the HR and that this compound induces cell death as well as primes defense in naive tissue. Two different mutants impaired in the pathogen-induced accumulation of sulforaphane displayed attenuated programmed cell death upon bacterial and oomycete effector recognition as well as decreased resistance to several isolates of the plant pathogen Hyaloperonospora arabidopsidis. Treatment with sulforaphane provided protection against a virulent H. arabidopsidis isolate. Glucosinolate breakdown products are recognized as antifeeding compounds toward insects and recently also as intracellular signaling and bacteriostatic molecules in Arabidopsis. The data presented here indicate that these compounds also trigger local defense responses in Arabidopsis tissue.
© 2015 American Society of Plant Biologists. All Rights Reserved.
Figures




References
-
- Agerbirk N, Olsen CE (2012) Glucosinolate structures in evolution. Phytochemistry 77: 16–45 - PubMed
-
- Andersson MX, Kourtchenko O, Dangl JL, Mackey D, Ellerström M (2006) Phospholipase-dependent signalling during the AvrRpm1- and AvrRpt2-induced disease resistance responses in Arabidopsis thaliana. Plant J 47: 947–959 - PubMed
-
- Bailey K, Cevik V, Holton N, Byrne-Richardson J, Sohn KH, Coates M, Woods-Tör A, Aksoy HM, Hughes L, Baxter L, et al. (2011) Molecular cloning of ATR5(Emoy2) from Hyaloperonospora arabidopsidis, an avirulence determinant that triggers RPP5-mediated defense in Arabidopsis. Mol Plant Microbe Interact 24: 827–838 - PubMed
-
- Barth C, Jander G (2006) Arabidopsis myrosinases TGG1 and TGG2 have redundant function in glucosinolate breakdown and insect defense. Plant J 46: 549–562 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

