Spinal fMRI reveals decreased descending inhibition during secondary mechanical hyperalgesia
- PMID: 25372292
- PMCID: PMC4221460
- DOI: 10.1371/journal.pone.0112325
Spinal fMRI reveals decreased descending inhibition during secondary mechanical hyperalgesia
Abstract
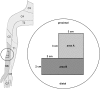
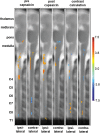
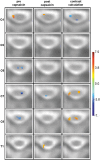
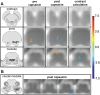
Mechanical hyperalgesia is one distressing symptom of neuropathic pain which is explained by central sensitization of the nociceptive system. This sensitization can be induced experimentally with the heat/capsaicin sensitization model. The aim was to investigate and compare spinal and supraspinal activation patterns of identical mechanical stimulation before and after sensitization using functional spinal magnetic resonance imaging (spinal fMRI). Sixteen healthy subjects (6 female, 10 male, mean age 27.2 ± 4.0 years) were investigated with mechanical stimulation of the C6 dermatome of the right forearm during spinal fMRI. Testing was always performed in the area outside of capsaicin application (i.e. area of secondary mechanical hyperalgesia). During slightly noxious mechanical stimulation before sensitization, activity was observed in ipsilateral dorsolateral pontine tegmentum (DLPT) which correlated with activity in ipsilateral spinal cord dorsal gray matter (dGM) suggesting activation of descending nociceptive inhibition. During secondary mechanical hyperalgesia, decreased activity was observed in bilateral DLPT, ipsilateral/midline rostral ventromedial medulla (RVM), and contralateral subnucleus reticularis dorsalis, which correlated with activity in ipsilateral dGM. Comparison of voxel-based activation patterns during mechanical stimulation before/after sensitization showed deactivations in RVM and activations in superficial ipsilateral dGM. This study revealed increased spinal activity and decreased activity in supraspinal centers involved in pain modulation (SRD, RVM, DLPT) during secondary mechanical hyperalgesia suggesting facilitation of nociception via decreased endogenous inhibition. Results should help prioritize approaches for further in vivo studies on pain processing and modulation in humans.
Conflict of interest statement
Figures

References
-
- Baron R, Binder A, Wasner G (2010) Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol 9: 807–819. - PubMed
-
- Simone DA, Sorkin LS, Oh U, Chung JM, Owens C, et al. (1991) Neurogenic hyperalgesia: central neural correlates in responses of spinothalamic tract neurons. J Neurophysiol 66: 228–246. - PubMed
-
- Petersen KL, Rowbotham MC (1999) A new human experimental pain model: the heat/capsaicin sensitization model. Neuroreport 10: 1511–1516. - PubMed
-
- Villanueva L, Lopez-Avila A, Le Bars D (2006) Ascending nociceptive pathways. In: Cervero F, Jensen TS, editors. Handbook of Clinical Neurology, Vol 81 (3rd series): Pain. Amsterdam: Elsevier B.V. pp. 93–102. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

