Caspase-1/ASC inflammasome-mediated activation of IL-1β-ROS-NF-κB pathway for control of Trypanosoma cruzi replication and survival is dispensable in NLRP3-/- macrophages
- PMID: 25372293
- PMCID: PMC4221042
- DOI: 10.1371/journal.pone.0111539
Caspase-1/ASC inflammasome-mediated activation of IL-1β-ROS-NF-κB pathway for control of Trypanosoma cruzi replication and survival is dispensable in NLRP3-/- macrophages
Abstract
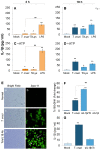
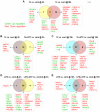
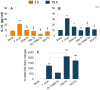
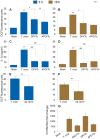
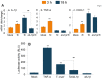
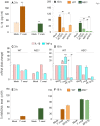
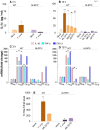
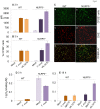
In this study, we have utilized wild-type (WT), ASC-/-, and NLRP3-/- macrophages and inhibition approaches to investigate the mechanisms of inflammasome activation and their role in Trypanosoma cruzi infection. We also probed human macrophages and analyzed published microarray datasets from human fibroblasts, and endothelial and smooth muscle cells for T. cruzi-induced changes in the expression genes included in the RT Profiler Human Inflammasome arrays. T. cruzi infection elicited a subdued and delayed activation of inflammasome-related gene expression and IL-1β production in mφs in comparison to LPS-treated controls. When WT and ASC-/- macrophages were treated with inhibitors of caspase-1, IL-1β, or NADPH oxidase, we found that IL-1β production by caspase-1/ASC inflammasome required reactive oxygen species (ROS) as a secondary signal. Moreover, IL-1β regulated NF-κB signaling of inflammatory cytokine gene expression and, subsequently, intracellular parasite replication in macrophages. NLRP3-/- macrophages, despite an inability to elicit IL-1β activation and inflammatory cytokine gene expression, exhibited a 4-fold decline in intracellular parasites in comparison to that noted in matched WT controls. NLRP3-/- macrophages were not refractory to T. cruzi, and instead exhibited a very high basal level of ROS (>100-fold higher than WT controls) that was maintained after infection in an IL-1β-independent manner and contributed to efficient parasite killing. We conclude that caspase-1/ASC inflammasomes play a significant role in the activation of IL-1β/ROS and NF-κB signaling of cytokine gene expression for T. cruzi control in human and mouse macrophages. However, NLRP3-mediated IL-1β/NFκB activation is dispensable and compensated for by ROS-mediated control of T. cruzi replication and survival in macrophages.
Conflict of interest statement
Figures
References
-
- World Health Organization (2010) Chagas disease: control and elimination. UNDP/World Bank/WHO. Available: http://apps.who.int/gb/ebwha/pdf_files/WHA63/A63_17-en.pdf. Accessed 2014 Sept 3
-
- Huang H, Chan J, Wittner M, Jelicks LA, Morris SA, et al. (1999) Expression of cardiac cytokines and inducible form of nitric oxide synthase (NOS2) in Trypanosoma cruzi-infected mice. J Mol Cell Cardiol 31: 75–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

