Citric acid-based hydroxyapatite composite scaffolds enhance calvarial regeneration
- PMID: 25372769
- PMCID: PMC4220725
- DOI: 10.1038/srep06912
Citric acid-based hydroxyapatite composite scaffolds enhance calvarial regeneration
Abstract
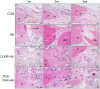
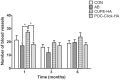
Citric acid-based polymer/hydroxyapatite composites (CABP-HAs) are a novel class of biomimetic composites that have recently attracted significant attention in tissue engineering. The objective of this study was to compare the efficacy of using two different CABP-HAs, poly (1,8-octanediol citrate)-click-HA (POC-Click-HA) and crosslinked urethane-doped polyester-HA (CUPE-HA) as an alternative to autologous tissue grafts in the repair of skeletal defects. CABP-HA disc-shaped scaffolds (65 wt.-% HA with 70% porosity) were used as bare implants without the addition of growth factors or cells to renovate 4 mm diameter rat calvarial defects (n = 72, n = 18 per group). Defects were either left empty (negative control group), or treated with CUPE-HA scaffolds, POC-Click-HA scaffolds, or autologous bone grafts (AB group). Radiological and histological data showed a significant enhancement of osteogenesis in defects treated with CUPE-HA scaffolds when compared to POC-Click-HA scaffolds. Both, POC-Click-HA and CUPE-HA scaffolds, resulted in enhanced bone mineral density, trabecular thickness, and angiogenesis when compared to the control groups at 1, 3, and 6 months post-trauma. These results show the potential of CABP-HA bare implants as biocompatible, osteogenic, and off-shelf-available options in the repair of orthopedic defects.
Figures






References
-
- Giannoudis P. V., Dinopoulos H. & Tsiridis E. Bone substitutes: an update. Injury 36 Suppl 3S20–27 (2005). - PubMed
-
- Watson J. T. Overview of biologics. J. Orthop. Trauma. 19, S14–16 (2005). - PubMed
-
- Friedlander A. H. The physiology, medical management and oral implications of menopause. J. Am. Dent. Assoc. 133, 73–81 (2002). - PubMed
-
- Bauer T. W. & Muschler G. F. Bone graft materials. An overview of the basic science. Clin. Orthop. Relat. R. 371, 10–27 (2000). - PubMed
-
- Acarturk T. O. & Hollinger J. O. Commercially available demineralized bone matrix compositions to regenerate calvarial critical-sized bone defects. Plast. Reconstr. Srug. 118, 862–873 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

