Optimization of crystallization conditions for biological macromolecules
- PMID: 25372810
- PMCID: PMC4231845
- DOI: 10.1107/S2053230X14019670
Optimization of crystallization conditions for biological macromolecules
Abstract
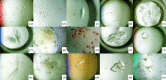
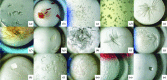
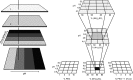
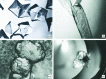
For the successful X-ray structure determination of macromolecules, it is first necessary to identify, usually by matrix screening, conditions that yield some sort of crystals. Initial crystals are frequently microcrystals or clusters, and often have unfavorable morphologies or yield poor diffraction intensities. It is therefore generally necessary to improve upon these initial conditions in order to obtain better crystals of sufficient quality for X-ray data collection. Even when the initial samples are suitable, often marginally, refinement of conditions is recommended in order to obtain the highest quality crystals that can be grown. The quality of an X-ray structure determination is directly correlated with the size and the perfection of the crystalline samples; thus, refinement of conditions should always be a primary component of crystal growth. The improvement process is referred to as optimization, and it entails sequential, incremental changes in the chemical parameters that influence crystallization, such as pH, ionic strength and precipitant concentration, as well as physical parameters such as temperature, sample volume and overall methodology. It also includes the application of some unique procedures and approaches, and the addition of novel components such as detergents, ligands or other small molecules that may enhance nucleation or crystal development. Here, an attempt is made to provide guidance on how optimization might best be applied to crystal-growth problems, and what parameters and factors might most profitably be explored to accelerate and achieve success.
Keywords: X-ray diffraction; additives; crystal growth; nucleation; pH; precipitants; proteins; strategy.
Figures

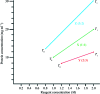
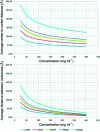
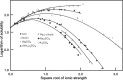





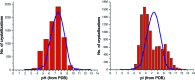




References
-
- Abergel, C. (2004). Spectacular improvement of X-ray diffraction through fast desiccation of protein crystals. Acta Cryst. D60, 1413–1416. - PubMed
-
- Adams, M. J., Haas, D. J., Jeffery, B. A., McPherson, A., Mermall, H. L., Rossmann, M. G., Schevitz, R. W. & Wonacott, A. J. (1969). Low resolution study of crystalline l-lactate dehydrogenase. J. Mol. Biol. 41, 159–188. - PubMed
-
- Adawy, A., Rebuffet, E., Törnroth-Horsefield, S., de Grip, W. J., van Enckevort, W. J. P. & Vlieg, E. (2012). High resolution protein crystals using an efficient convection-free geometry. Cryst. Growth Des. 13, 775–781.
-
- Arakawa, T. & Timasheff, S. N. (1985). Calculation of the partial specific volume of proteins in concentrated salt and amino acid solutions. Methods Enzymol. 117, 60–65. - PubMed
-
- Astier, J.-P. & Veesler, S. (2008). Using temperature to crystallize proteins: a mini-review. Cryst. Growth Des. 8, 4215–4219.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

