Lymphocyte Activation Gene 3 (LAG-3) modulates the ability of CD4 T-cells to be suppressed in vivo
- PMID: 25372844
- PMCID: PMC4220939
- DOI: 10.1371/journal.pone.0109080
Lymphocyte Activation Gene 3 (LAG-3) modulates the ability of CD4 T-cells to be suppressed in vivo
Abstract
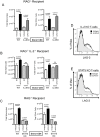
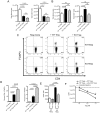
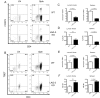
Lymphocyte Activation Gene - 3 (LAG-3) is an immune checkpoint molecule that regulates both T-cell activation and homeostasis. However, the molecular mechanisms underlying LAG-3's function are generally unknown. Using a model in which LAG-3 blockade or absence reliably augmented homeostatic proliferation in vivo, we found that IL-2 and STAT5 are critical for LAG-3 function. Similarly, LAG-3 blockade was ineffective in the absence of regulatory T-cells (Treg), suggesting an important role for LAG-3 in either the responsiveness of conventional T-cells (Tconv) to regulation, or a relative defect in the ability of LAG-3 KO regulatory T-cells (Treg) to suppress the proliferation of Tconv. In this model, LAG-3 KO Treg suppressed proliferation in a manner fairly similar to wild-type (WT) Treg, but LAG-3 KO Tconv were relatively resistant to suppression. Further studies also identified a role for LAG-3 in the induction/expansion of Treg. Finally, we found that LAG-3 blockade (or knockout) led to a relative skewing of naïve CD4 T-cells toward a TH1 phenotype both in vitro and in in vivo. Together, these data suggest that LAG-3 expression on Tconv cells makes them more susceptible to Treg based suppression, and also regulates the development of a TH1 T-cell response.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

