Emerging importance of oxidative stress in regulating striated muscle elasticity
- PMID: 25373878
- PMCID: PMC4352196
- DOI: 10.1007/s10974-014-9392-y
Emerging importance of oxidative stress in regulating striated muscle elasticity
Abstract
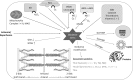
The contractile function of striated muscle cells is altered by oxidative/nitrosative stress, which can be observed under physiological conditions but also in diseases like heart failure or muscular dystrophy. Oxidative stress causes oxidative modifications of myofilament proteins and can impair myocyte contractility. Recent evidence also suggests an important effect of oxidative stress on muscle elasticity and passive stiffness via modifications of the giant protein titin. In this review we provide a short overview of known oxidative modifications in thin and thick filament proteins and then discuss in more detail those oxidative stress-related modifications altering titin stiffness directly or indirectly. Direct modifications of titin include reversible disulfide bonding within the cardiac-specific N2-Bus domain, which increases titin stiffness, and reversible S-glutathionylation of cryptic cysteines in immunoglobulin-like domains, which only takes place after the domains have unfolded and which reduces titin stiffness in cardiac and skeletal muscle. Indirect effects of oxidative stress on titin can occur via reversible modifications of protein kinase signalling pathways (especially the NO-cGMP-PKG axis), which alter the phosphorylation level of certain disordered titin domains and thereby modulate titin stiffness. Oxidative stress also activates proteases such as matrix-metalloproteinase-2 and (indirectly via increasing the intracellular calcium level) calpain-1, both of which cleave titin to irreversibly reduce titin-based stiffness. Although some of these mechanisms require confirmation in the in vivo setting, there is evidence that oxidative stress-related modifications of titin are relevant in the context of biomarker design and represent potential targets for therapeutic intervention in some forms of muscle and heart disease.
Figures

References
-
- Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88:287–332. - PubMed
-
- Andrade FH, Reid MB, Westerblad H. Contractile response of skeletal muscle to low peroxide concentrations: myofibrillar calcium sensitivity as a likely target for redox-modulation. FASEB J. 2001;15:309–311. - PubMed
-
- Arcaro A, Lembo G, Tocchetti CG. Nitroxyl (HNO) for treatment of acute heart failure. Curr Heart Fail Rep. 2014;11:227–235. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

