LPS from P. gingivalis and hypoxia increases oxidative stress in periodontal ligament fibroblasts and contributes to periodontitis
- PMID: 25374447
- PMCID: PMC4211166
- DOI: 10.1155/2014/986264
LPS from P. gingivalis and hypoxia increases oxidative stress in periodontal ligament fibroblasts and contributes to periodontitis
Abstract
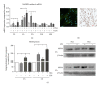
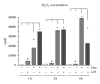
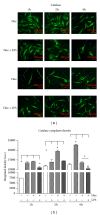
Oxidative stress is characterized by an accumulation of reactive oxygen species (ROS) and plays a key role in the progression of inflammatory diseases. We hypothesize that hypoxic and inflammatory events induce oxidative stress in the periodontal ligament (PDL) by activating NOX4. Human primary PDL fibroblasts were stimulated with lipopolysaccharide from Porphyromonas gingivalis (LPS-PG), a periodontal pathogen bacterium under normoxic and hypoxic conditions. By quantitative PCR, immunoblot, immunostaining, and a specific ROS assay we determined the amount of NOX4, ROS, and several redox systems. Healthy and inflamed periodontal tissues were collected to evaluate NOX4 and redox systems by immunohistochemistry. We found significantly increased NOX4 levels after hypoxic or inflammatory stimulation in PDL cells (P < 0.001) which was even more pronounced after combination of the stimuli. This was accompanied by a significant upregulation of ROS and catalase (P < 0.001). However, prolonged incubation with both stimuli induced a reduction of catalase indicating a collapse of the protective machinery favoring ROS increase and the progression of inflammatory oral diseases. Analysis of inflamed tissues confirmed our hypothesis. In conclusion, we demonstrated that the interplay of NOX4 and redox systems is crucial for ROS formation which plays a pivotal role during oral diseases.
Figures



References
-
- Poyton RO, Ball KA, Castello PR. Mitochondrial generation of free radicals and hypoxic signaling. Trends in Endocrinology and Metabolism. 2009;20(7):332–340. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

