The connexin43 mimetic peptide Gap19 inhibits hemichannels without altering gap junctional communication in astrocytes
- PMID: 25374505
- PMCID: PMC4204617
- DOI: 10.3389/fncel.2014.00306
The connexin43 mimetic peptide Gap19 inhibits hemichannels without altering gap junctional communication in astrocytes
Abstract
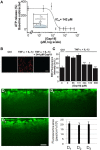
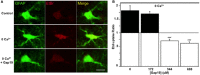
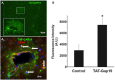
In the brain, astrocytes represent the cellular population that expresses the highest amount of connexins (Cxs). This family of membrane proteins is the molecular constituent of gap junction channels and hemichannels that provide pathways for direct cytoplasm-to-cytoplasm and inside-out exchange, respectively. Both types of Cx channels are permeable to ions and small signaling molecules allowing astrocytes to establish dynamic interactions with neurons. So far, most pharmacological approaches currently available do not distinguish between these two channel functions, stressing the need to develop new specific molecular tools. In astrocytes two major Cxs are expressed, Cx43 and Cx30, and there is now evidence indicating that at least Cx43 operates as a gap junction channel as well as a hemichannel in these cells. Based on studies in primary cultures as well as in acute hippocampal slices, we report here that Gap19, a nonapeptide derived from the cytoplasmic loop of Cx43, inhibits astroglial Cx43 hemichannels in a dose-dependent manner, without affecting gap junction channels. This peptide, which not only selectively inhibits hemichannels but is also specific for Cx43, can be delivered in vivo in mice as TAT-Gap19, and displays penetration into the brain parenchyma. As a result, Gap19 combined with other tools opens up new avenues to decipher the role of Cx43 hemichannels in interactions between astrocytes and neurons in physiological as well as pathological situations.
Keywords: astroglia; connexins; gap junctions; glial cells; mimetic peptide.
Figures

References
-
- D'hondt C., Iyyathurai J., Wang N., Gourdie R. G., Himpens B., Leybaert L., et al. . (2013). Negatively charged residues (Asp378 and Asp379) in the last ten amino acids of the C-terminal tail of Cx43 hemichannels are essential for loop/tail interactions. Biochem. Biophys. Res. Commun. 432, 707–712. 10.1016/j.bbrc.2013.01.066 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

