Expanding use of new oral anticoagulants
- PMID: 25374671
- PMCID: PMC4191267
- DOI: 10.12703/P6-93
Expanding use of new oral anticoagulants
Abstract
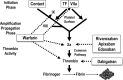
New, non-vitamin K antagonist oral anticoagulants (NOACs) have been developed to overcome the limitations of warfarin. These include dabigatran, which inhibits thrombin, and rivaroxaban, apixaban, and edoxaban, which inhibit factor Xa. In the US, rivaroxaban and apixaban are licensed for thromboprophylaxis after elective hip or knee arthroplasty, and rivaroxaban and dabigatran are approved for treatment of venous thromboembolism. Dabigatran, rivaroxaban, and apixaban also are licensed for stroke prevention in eligible patients with atrial fibrillation. Designed to be given in fixed doses without routine coagulation monitoring, the NOACs are more convenient to administer than warfarin. Phase III clinical trials have shown that the NOACs are at least as effective as warfarin and are associated with less intracranial bleeding. This article compares the pharmacological properties of the NOACs with those of warfarin, describes the clinical trial data with the NOACs in the approved indications, outlines the unmet medical needs that the NOACs address, highlights the potential limitations of the NOACs, and provides guidance on the optimal use of the NOACs.
Figures
References
-
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G, American College of Chest Physicians Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th Ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;143:44S–68S. doi: 10.1378/chest.11-2292. - DOI - PMC - PubMed
-
- Colwell CW, Jr, Collis DK, Paulson R, McCutchen JW, Bigler GT, Lutz S, Hardwick ME. Comparison of enoxaparin and warfarin for the prevention of venous thromboembolic disease after total hip arthroplasty. Evaluation during hospitalization and three months after discharge. J Bone Joint Surg Am. 1999;81:932–40. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources