Protocolized versus non-protocolized weaning for reducing the duration of mechanical ventilation in critically ill adult patients
- PMID: 25375085
- PMCID: PMC6517015
- DOI: 10.1002/14651858.CD006904.pub3
Protocolized versus non-protocolized weaning for reducing the duration of mechanical ventilation in critically ill adult patients
Abstract
Background: This is an update of a review last published in Issue 5, 2010, of The Cochrane Library. Reducing weaning time is desirable in minimizing potential complications from mechanical ventilation. Standardized weaning protocols are purported to reduce time spent on mechanical ventilation. However, evidence supporting their use in clinical practice is inconsistent.
Objectives: The first objective of this review was to compare the total duration of mechanical ventilation of critically ill adults who were weaned using protocols versus usual (non-protocolized) practice.The second objective was to ascertain differences between protocolized and non-protocolized weaning in outcomes measuring weaning duration, harm (adverse events) and resource use (intensive care unit (ICU) and hospital length of stay, cost).The third objective was to explore, using subgroup analyses, variations in outcomes by type of ICU, type of protocol and approach to delivering the protocol (professional-led or computer-driven).
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library Issue 1, 2014), MEDLINE (1950 to January 2014), EMBASE (1988 to January 2014), CINAHL (1937 to January 2014), LILACS (1982 to January 2014), ISI Web of Science and ISI Conference Proceedings (1970 to February 2014), and reference lists of articles. We did not apply language restrictions. The original search was performed in January 2010 and updated in January 2014.
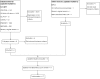
Selection criteria: We included randomized controlled trials (RCTs) and quasi-RCTs of protocolized weaning versus non-protocolized weaning from mechanical ventilation in critically ill adults.
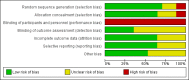
Data collection and analysis: Two authors independently assessed trial quality and extracted data. We performed a priori subgroup and sensitivity analyses. We contacted study authors for additional information.
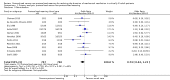
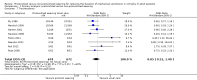
Main results: We included 17 trials (with 2434 patients) in this updated review. The original review included 11 trials. The total geometric mean duration of mechanical ventilation in the protocolized weaning group was on average reduced by 26% compared with the usual care group (N = 14 trials, 95% confidence interval (CI) 13% to 37%, P = 0.0002). Reductions were most likely to occur in medical, surgical and mixed ICUs, but not in neurosurgical ICUs. Weaning duration was reduced by 70% (N = 8 trials, 95% CI 27% to 88%, P = 0.009); and ICU length of stay by 11% (N = 9 trials, 95% CI 3% to 19%, P = 0.01). There was significant heterogeneity among studies for total duration of mechanical ventilation (I(2) = 67%, P < 0.0001) and weaning duration (I(2) = 97%, P < 0.00001), which could not be explained by subgroup analyses based on type of unit or type of approach.
Authors' conclusions: There is evidence of reduced duration of mechanical ventilation, weaning duration and ICU length of stay with use of standardized weaning protocols. Reductions are most likely to occur in medical, surgical and mixed ICUs, but not in neurosurgical ICUs. However, significant heterogeneity among studies indicates caution in generalizing results. Some study authors suggest that organizational context may influence outcomes, however these factors were not considered in all included studies and could not be evaluated. Future trials should consider an evaluation of the process of intervention delivery to distinguish between intervention and implementation effects. There is an important need for further development and research in the neurosurgical population.
Conflict of interest statement
Bronagh Blackwood: none known.
Karen EA Burns: holds a CAD 5000 travel bursary from Draeger Medical Inc. (Canada) for the purpose of conducting site visits to participating centres in the WEAN Study. The WEAN study is not included in this Cochrane review. (The WEAN Study is an investigator‐initiated trial comparing SmartCare™ and protocolized weaning, for which the co‐principal investigator (Dr Burns) obtained funding from peer review, non‐industry sources for implementation. Draeger Medical Inc. provided ventilators and ventilator upgrades for the WEAN study and a central randomization system using electronic mail correspondence (Draeger Medical, Germany). Draeger Medical was not involved in any aspects of study design and oversight, data management or data analysis).
Chris R Cardwell: none known.
Peter O'Halloran: none known.
Figures





















Update of
-
Protocolized versus non-protocolized weaning for reducing the duration of mechanical ventilation in critically ill adult patients.Cochrane Database Syst Rev. 2010 May 12;(5):CD006904. doi: 10.1002/14651858.CD006904.pub2. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2014 Nov 06;(11):CD006904. doi: 10.1002/14651858.CD006904.pub3. PMID: 20464747 Updated.
References
References to studies included in this review
Chaiwat 2010 {published data only (unpublished sought but not used)}
-
- Chaiwat O, Sarima N, Niyompanitpattana K, Komoltri C, Udomphorn Y, Kongsayreepong S. Protocol‐directed vs. physician‐directed weaning from ventilator in intra‐abdominal surgical patients. Journal of Medical Association of Thailand 2010;93(8):930‐6. - PubMed
de Carvalho Oliveira 2002 {published and unpublished data}
-
- Carvalho Oliveira LR, Jose A, Dias EC, dos Santos VLA, Chiavone PA. Weaning protocol for mechanical ventilation: effects of its use in an intensive care unit. A controlled, prospective and randomized trial. Revista Brasileira Terapia Intensiva 2002;14(1):22‐32.
Ely 1996 {published and unpublished data}
-
- Ely EW, Baker AM, Dunagan DP, Burke HL, Smith AC, Kelly PT, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. New England Journal of Medicine 1996;335(25):1864‐9. [PUBMED: 8948561] - PubMed
Fan 2013 {published data only}
-
- Fan L, Su H, Zhang Y, Zhang Y, Gao D, Ye H, et al. A randomized controlled trial of protocol‐directed versus physician‐directed weaning from mechanical ventilation in neuro‐critical patients. Chinese Journal of Neurology 2013;46(5):320‐3.
Kollef 1997 {published and unpublished data}
-
- Kollef MH, Shapiro SD, Silver P, John RE, Prentice D, Sauer S, et al. A randomized, controlled trial of protocol‐directed versus physician‐directed weaning from mechanical ventilation. Critical Care Medicine 1997;25(4):567‐74. [PUBMED: 9142019 ] - PubMed
Krishnan 2004 {published and unpublished data}
-
- Krishnan JA, Moore D, Robeson C, Rand CS, Fessler HE. A prospective, controlled trial of a protocol‐based strategy to discontinue mechanical ventilation. American Journal of Respiratory and Critical Care Medicine 2004;169(6):673‐8. [PUBMED: 14726421] - PubMed
Marelich 2000 {published and unpublished data}
-
- Marelich GP, Murin S, Battistella F, Inciardi J, Vierra T, Roby M. Protocol weaning of mechanical ventilation in medical and surgical patients by respiratory care practitioners and nurses: effect on weaning time and incidence of ventilator‐associated pneumonia. Chest 2000;118(2):459‐67. [PUBMED: 10936141] - PubMed
Namen 2001 {published and unpublished data}
-
- Namen AM, Ely EW, Tatter SB, Case LD, Lucia MA, Smith A, et al. Predictors of successful extubation in neurosurgical patients. American Journal of Respiratory and Critical Care Medicine 2001;163(3 Pt 1):658‐64. [PUBMED: 11254520 ] - PubMed
Navalesi 2008 {published and unpublished data}
-
- Navalesi P, Frigerio P, Moretti MP, Sommariva M, Vesconi S, Baiardi P, et al. Rate of reintubation in mechanically ventilated neurosurgical and neurologic patients: evaluation of a systematic approach to weaning and extubation. Critical Care Medicine 2008;36(11):2986‐92. [PUBMED: 18824909] - PubMed
Ogica 2007 {published and unpublished data}
-
- Ogica A, Droc G, Tomescu D, Popescu H, Tulbure D. Weaning from mechanical ventilation: protocol vs physician decision. European Journal of Anaesthesiology 2007;24:147‐8.
Piotto 2011 {published and unpublished data}
-
- Piotto RF, Maia LN, Machado MN, Orrico SP. Effects of the use of mechanical ventilation weaning protocol in the coronary care unit: randomized study. Revista Brasileira de Cirurgia Cardiovascular 2011;26(2):213‐221. - PubMed
Reardon 2011 {unpublished data only}
-
- Reardon CC, Walkey AJ. Clinical trial of a computer‐driven weaning system for patients requiring mechanical ventilation. http://www.clinicaltrials.gov/ 2011.
Roh 2012 {published and unpublished data}
-
- Roh JH, Synn A, Lim C, Suh HJ, Hong S, Huh JW, et al. A weaning protocol administered by critical care nurses for the weaning of patients from mechanical ventilation. Journal of Critical Care 2012;27(6):549‐55. - PubMed
Rose 2008 {published data only}
-
- Rose L, Presneill JJ, Johnston L, Cade JF. A randomised, controlled trial of conventional versus automated weaning from mechanical ventilation using SmartCare TM/PS. Intensive Care Medicine 2008;34(10):1788‐95. [PUBMED: 18575843 ] - PubMed
Simeone 2002 {published and unpublished data}
-
- Simeone F, Biagioli B, Scolletta S, Marullo ACM, Marchetti L, Caciorgna M, et al. Optimization of mechanical ventilation support following cardiac surgery. Journal of Cardiovascular Surgery 2002;43(5):633‐41. [PUBMED: 12386574 ] - PubMed
Stahl 2009 {published and unpublished data}
-
- Stahl C, Dahmen G, Ziegler A, Muhl E. Comparison of automated protocol‐based versus non‐protocol‐based physician‐directed weaning from mechanical ventilation. Intensivmedizin und Notfallmedizin 2009;46(6):441‐6. [DOI: 10.1007/s00390-009-0061-0] - DOI
Strickland 1993 {published data only}
-
- Strickland Jr JH, Hasson JH. A computer‐controlled, ventilator weaning system. A clinical trial. Chest 1993;103(4):1220‐6. [PUBMED: 8131469] - PubMed
References to studies excluded from this review
Beale 2008 {unpublished data only}
-
- Beale R. Comparison of an automated weaning programme and a standard clinical weaning protocol for weaning critically ill patients. ISRCTN Register.
Donglemans 2009 {published data only}
-
- Donglemans DA, Veelo DP, Paulus F, Mol BA, Korevar JC, Kudoga A, et al. Weaning automation with adaptive support ventilation: a randomized controlled trial in cardiothoracic surgery patients. Anesthesia and Analgesia 2009;108(2):565‐71. - PubMed
East 1999 {published and unpublished data}
-
- East TD, Heermann LK, Bradshaw RL, Lugo A, Sailors RM, Ershler L, et al. Efficacy of computerized decision support for mechanical ventilation: results of a prospective multi‐center randomized trial. Journal of the American Medical Informatics Association 1999;Suppl:251‐5. [PUBMED: 10566359 ] - PMC - PubMed
Gnanapandithan 2011 {published data only}
-
- Gnanapandithan K, Agarwal R, Aggarwal AN, Gupta D. Weaning by gradual pressure support (PS) reduction without an initial spontaneous breathing trial (SBT) versus PS‐supported SBT: a pilot study. Revista Portuguesa de Pneumologia 2011;17(6):244‐52. - PubMed
Lellouche 2006 {published data only}
Liu 2013 {published data only}
Ma 2010a {published data only}
-
- Ma YM, Liu YN, Pan L. The effect of spontaneous breathing trial on weaning from ventilators. Chinese Journal of Tuberculosis and Respiratory Diseases 2010;33(3):179‐182. - PubMed
Ma 2010b {published data only (unpublished sought but not used)}
-
- Ma YJ, Yang XJ, Cao XY, Ma XG. Comparison of computer‐driven weaning and physician‐directed weaning from mechanical ventilation. Chinese Journal of Tuberculosis and Respiratory Diseases 2010;33(3):174‐178. - PubMed
McKinley 2001 {published data only}
-
- McKinley BA, Moore FA, Sailors RM, Cocanour CS, Marquez A, Wright RK, et al. Computerized decision support for mechanical ventilation of trauma induced ARDS: results of a randomized clinical trial. The Journal of Trauma Injury, Infection and Critical Care 2001;50(3):415‐24. [PUBMED: 11265020] - PubMed
NCT00157287 {unpublished data only}
-
- NCT00157287. A cluster randomized trial to improve weaning and extubation from mechanical ventilation in community hospitals. clinicaltrials.gov/ct2/show/NCT00157287 (accessed 30 January 2014).
NCT00445289 {unpublished data only}
-
- NCT00445289. Automatic control of pressure support ventilation in surgical intensive care units. http://clinicaltrials.gov/show/NCT00445289 (accessed 30 January 2014).
NCT00502489 {unpublished data only}
-
- NCT00502489. Computer driven management of weaning following prolonged mechanical ventilation. clinicaltrials.gov/show/NCT00502489 (accessed 30 January 2014).
Taniguchi 2009 {published data only}
Vaschetto 2011 {published data only (unpublished sought but not used)}
-
- Vaschetto R, Levati A, Boggero A, Gollo Y, Moretti MP, Sommariva M, et al. Does a weaning protocol facilitate liberation from mechanical ventilation in tracheotomized brain‐injured patients?. Intensive Care Medicine. 2011; Vol. 37:S6.
Additional references
Aitken 2012
-
- Aitken LM, Bucknall T, Kent B, Mitchell M, Burmeister E, Keogh SJ. Protocol directed sedation versus non‐protocol directed sedation to reduce duration of mechanical ventilation in mechanically ventilated intensive care patients. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD009771] - DOI - PubMed
Alia 2000
Blackwood 2006
-
- Blackwood B. Methodological issues in evaluating complex healthcare interventions. Journal of Advanced Nursing 2006;54(5):612‐22. [PUBMED: 16722959 ] - PubMed
Blackwood 2013
-
- Blackwood B, Murray M, Chisakuta A, Cardwell CR, O’Halloran P. Protocolized versus non‐protocolized weaning for reducing the duration of invasive mechanical ventilation in critically ill paediatric patients. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD009082.pub2] - DOI - PMC - PubMed
Blackwood 2014
-
- Blackwood B, Clarke M, McAuley DF, McGuigan P, Marshall JC, Rose L. How outcomes are defined in clinical trials of mechanically ventilated adults and children. American Journal of Respiratory and Critical Care Medicine 2014;189(8):886‐93. [PUBMED: 24512505] - PubMed
Bland 1996
Boles 2007
-
- Boles JM, Bion J, Connors A, Herridge M, Marsh B, Melot C, et al. Weaning from mechanical ventilation. European Respiratory Journal 2007;29(5):1033‐56. [PUBMED: 17470624] - PubMed
Brochard 1994
-
- Brochard L, Rauss A, Benito S, Conti G, Mancebo J, Rekik N, et al. Comparison of three methods of gradual withdrawal from ventilatory support during weaning from mechanical ventilation. American Journal of Respiratory and Critical Care Medicine 1994;150(4):896‐903. [PUBMED: 7921460] - PubMed
Bucknall 2008
Burns 2008
-
- Burns KEA, Lellouche F, Lessard MR. Automating the weaning process with advanced closed‐loop systems. Intensive Care Medicine 2008;34(10):1757‐65. [PUBMED: 18521570 ] - PubMed
Cook 2000
Egger 1997
Ellis 2012
Esen 1992
-
- Esen F, Denkel T, Telci L, Kesecioglu J, Tütüncü AS, Akpir K, et al. Comparison of pressure support ventilation (PSV) and intermittent mandatory ventilation (IMV) during weaning in patients with acute respiratory failure. Advances in Experimental Medical Biology 1992;317:371‐6. [PUBMED: 1288147] - PubMed
Esteban 1995
-
- Esteban A, Frutos F, Tobin MJ, Alia I, Solsona JF, Valverdu I, et al. A comparison of four methods of weaning patients from mechanical ventilation. Spanish Lung Failure Collaborative Group. New England Journal of Medicine 1995;332(6):345‐50. [MEDLINE: ] - PubMed
Esteban 2008
-
- Esteban A, Ferguson ND, Meade MO, Frutos‐Vivar F, Apezteguia C, Brochard L, et al. Evolution of mechanical ventilation in response to clinical research. American Journal of Respiratory and Critical Care Medicine 2008;177(2):170‐7. [MEDLINE: ] - PubMed
Girard 2008
-
- Girard TD, Kress JP, Fuchs BD, Thomason JW, Schweickert WD, Pun BT, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet 2008;371(9607):126‐34. [DOI: 10.1016/S0140-6736(08)60105-1.] - DOI - PubMed
Guyatt 2008
-
- Guyatt GH, Oxman AD, Kunz R, Falck‐Ytter Y, Vist GE, Liberati A, et al. Rating quality of evidence and strength of recommendations: going from evidence to recommendations. BMJ 2008;36:1049‐51. [MEDLINE: ]
Hendrix 2006
-
- Hendrix H, Kaiser ME, Yusen RD, Merk J. A randomized trial of automated versus conventional protocol‐driven weaning from mechanical ventilation following coronary artery bypass surgery. European Journal of Cardio‐thoracic Surgery 2006;29(6):957‐63. [PUBMED: 16520042] - PubMed
Hess 2011
Higgins 2002
-
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [PUBMED: 12111919] - PubMed
Higgins 2008
Higgins 2011a
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hozo 2005
Jordan 2012
-
- Jordan J, Rose L, Noyes J, Dainty KN, Blackwood B. Factors that impact on the use of mechanical ventilation weaning protocols in critically ill adults and children: a qualitative evidence‐synthesis. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD009851] - DOI - PMC - PubMed
Kubler 2013
Levine 2008
-
- Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. New England Journal of Medicine 2008;358(13):1327‐35. [PUBMED: 18367735 ] - PubMed
MacIntyre 2001
-
- MacIntyre NR, Cook DJ, Ely EW, Epstein SK, Fink JB, Heffner JE, et al. Evidence‐based guidelines for weaning and discontinuing ventilatory support: a collective task force facilitated by the American College of Chest Physicians, the American Association for Respiratory Care, and the American College of Critical Care Medicine. Chest 2001;120 Suppl:375‐95. - PubMed
Mancebo 1996
-
- Mancebo J. Weaning from mechanical ventilation. European Respiratory Journal 1996;9(9):1923‐31. [PUBMED: 8880113] - PubMed
Marini 1995
-
- Marini JJ. Weaning techniques and protocols. Respiratory Care 1995;40(3):233‐8. [ISSN 0098‐9142]
Mehta 2008
-
- Mehta S, Burry L, Martinez‐Motta JC, Stewart TE, Hallett D, McDonald E, Clarke F, Macdonald R, Granton J, Matte A, Wong C, Suri A, Cook DJ, Canadian Critical Care Trials Group. A randomized trial of daily awakening in critically ill patients managed with a sedation protocol: a pilot trial. Critical Care Medicine 2008;36(7):2092‐9. [DOI: 10.1097/CCM.0b013e31817bff85] - DOI - PubMed
Mehta 2012
-
- Mehta S, Burry L, Cooke D, Fergusson D, Steinberg M, Granton J, et al. Daily sedation interruption in mechanically ventilated critically ill patients cared for with a sedation protocol: a randomized controlled trial. Journal of American Medical Association 2012;308(19):1985‐92. [DOI: 10.1001/jama.2012.13872] - DOI - PubMed
MRC 2008
-
- Medical Research Council. Developing and evaluating complex interventions: new guidance. www.mrc.ac.uk/complexinterventionsguidance (accessed 30 January 2014).
Murtagh 2007
-
- Murtagh D, Baum N. Protocols put more pennies in your practice pockets or protocols lead to practice improvement. www. neilbaum.com/articles/mkt_protocols.html (accessed 19 August 2007).
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rose 2007
-
- Rose L, Presneill JJ, Cade JF. Update in computer‐driven weaning from mechanical ventilation. Aneasthesia and Intensive Care 2007;35(2):213‐21. [PUBMED: 17444311] - PubMed
Rose 2011
Rose 2013
-
- Rose L, Schultz MJ, Cardwell CR, Jouvet P, McAuley DF, Blackwood B. Automated versus non‐automated weaning for reducing the duration of mechanical ventilation for critically ill adults and children. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD009235.pub2] - DOI
Sahn 1973
-
- Sahn SA, Lakshminarayan S. Bedside criteria for discontinuation of mechanical ventilation. Chest 1973;63(6):1002‐5. [PUBMED: 4514488] - PubMed
Stroetz 1995
-
- Stroetz RW, Hubmayer RD. Tidal volume maintenance during weaning with pressure support. American Journal of Respiratory and Critical Care Medicine 1995;152(3):1034‐40. [PUBMED: 7663780] - PubMed
References to other published versions of this review
Blackwood 2008
-
- Blackwood B, Alderdice F, Burns KE, Cardwell CR, Lavery G, O'Halloran P. Protocolized versus non‐protocolized weaning for reducing the duration of mechanical ventilation in critically ill adult patients. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD006904] - DOI - PubMed
Blackwood 2010
-
- Blackwood B, Alderdice F, Burns KE, Cardwell CR, Lavery G, O’Halloran P. Protocolized versus non‐protocolized weaning for reducing the duration of mechanical ventilation in critically ill adult patients. Cochrane Database of Systematic Reviews 2010, Issue 5. [DOI: 10.1002/14651858.CD006904.pub2] - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

