IFN-γ induces aberrant CD49b⁺ NK cell recruitment through regulating CX3CL1: a novel mechanism by which IFN-γ provokes pregnancy failure
- PMID: 25375377
- PMCID: PMC4260728
- DOI: 10.1038/cddis.2014.470
IFN-γ induces aberrant CD49b⁺ NK cell recruitment through regulating CX3CL1: a novel mechanism by which IFN-γ provokes pregnancy failure
Abstract
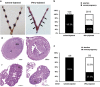
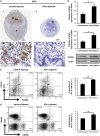
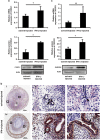
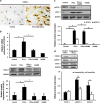
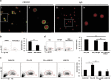
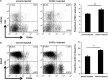
Interferon-γ (IFN-γ), a pleiotropic lymphokine, has important regulatory effects on many cell types. Although IFN-γ is essential for the initiation of uterine vascular modifications and maintenance of decidual integrity, IFN-γ administration can also cause pregnancy failure in many species. However, little is known about the effector mechanisms involved. In this study, using an IFN-γ-induced abortion mouse model, we reported that no Dolichos biflorus agglutinin lectin-positive uterine natural killer (uNK) cells were observed in the uteri from IFN-γ-induced abortion mice. By contrast, the percentage of CD3(-)CD49b(+) NK cells in the uterus and blood from a foetal resorption group was significantly higher than that of the control group. Similarly, significantly upregulated expression of CD49b (a pan-NK cell marker), CX3CL1 and CX3CR1 (CX3CL1 receptor) was detected in the uteri of IFN-γ-induced abortion mice. Using isolated uterine stromal cells, we showed that upregulated expression of CX3CL1 by IFN-γ was dependent on a Janus family kinase 2-signal transducers and activators of transcription 1 (JAK2-STAT1) pathway. We further demonstrated the chemotactic activity of CX3CL1 in uterine stromal cell conditioned medium on primary splenic NK cells. Finally, we observed increased recruitment of CD49b(+) NK cells into the endometrium after exogenous CX3CL1 administration. Collectively, our findings indicate that IFN-γ can significantly increase uterine CX3CL1 expression via activation of the JAK2-STAT1 pathway, thus inducing CD49b(+) NK cell uterine homing, and eventually provoke foetal loss. Thus, we provide a new line of evidence correlating the deleterious effects of IFN-γ on pregnancy with the aberrant regulation of CX3CL1 and CD49b(+) NK cells.
Figures
References
-
- Liu Z, Chen Y, Yang Y, Peng JP. The effect on MHC class II expression and apoptosis in placenta by IFNgamma administration. Contraception. 2002;65:177–184. - PubMed
-
- Chaouat G, Menu E, Clark DA, Dy M, Minkowski M, Wegmann TG. Control of fetal survival in CBAxDBA/2 mice by lymphokine therapy. J Reprod Fertil. 1990;89:447–458. - PubMed
-
- Sun QH, Peng JP, Xia HF, Yang Y, Liu ML. Effect on expression of RT1-A and RT1-DM molecules of treatment with interferon-gamma at the maternal–fetal interface of pregnant rats. Hum Reprod. 2005;20:2639–2647. - PubMed
-
- Liu Z, Sun QH, Yang Y, Liu JM, Peng JP. Effect of IFNgamma on caspase-3, Bcl-2 and Bax expression, and apoptosis in rabbit placenta. Cytokine. 2003;24:201–209. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

