Gastrointestinal adverse events of glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a systematic review and network meta-analysis
- PMID: 25375397
- PMCID: PMC4290796
- DOI: 10.1089/dia.2014.0188
Gastrointestinal adverse events of glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a systematic review and network meta-analysis
Abstract
Introduction: Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are a new class of drugs used in the treatment of type 2 diabetes mellitus (T2DM). Gastrointestinal (GI) adverse events (AEs) are the most frequently reported treatment-related AEs for GLP-1 RAs. We aim to evaluate the effect of GLP-1 RAs on the incidence of GI AEs of T2DM.
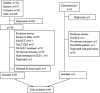
Materials and methods: The overview of the GI events of GLP-1 RAs has been performed on relevant publications through the literature search, such as MEDLINE, EMBASE, Cochrane Library, and ClinicalTrials.gov The manufacturer was contacted regarding unpublished data. We analyzed direct and indirect comparisons of different treatments using Bayesian network meta-analysis.
Results: Taspoglutide 30 mg once weekly (TAS30QW) and lixisenatide 30 μg twice daily (LIX30BID) were ranked the top two drugs in terms of GI AEs versus placebo. The odds ratios of nausea and vomiting for TAS30QW were 11.8 (95% confidence interval [CI], 2.89, 46.9) and 51.7 (95% CI, 7.07, 415), respectively, and that of diarrhea was 4.93 (95% CI, 1.75, 14.7) for LIX30BID.
Conclusions: Our study found all GLP-1 RA dose regimens significantly increased the incidence of GI AEs, compared with placebo or conventional treatment. The occurrence of GI AEs was different with diverse dose regimens of GLP-1 RAs. TAS30QW had the maximum probability to occur nausea and vomiting, whereas LIX30BID had the maximum probability to cause development of diarrhea versus other treatments.
Figures


References
-
- Whiting DR, Guariguata L, Weil C, et al. : IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 2011;94:311–321 - PubMed
-
- Levetan C: Oral antidiabetic agents in type 2 diabetes. Curr Med Res Opin 2007;23:945–952 - PubMed
-
- Hansen KB, Knop FK, Holst JJ, et al. : Treatment of type 2 diabetes with glucagon-like peptide-1 receptor agonists. Int J Clin Pract 2009;63:1154–1160 - PubMed
-
- Drucker DJ, Nauck MA: The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006;368:1696–1705 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

