Efficient uptake of blood-borne BK and JC polyomavirus-like particles in endothelial cells of liver sinusoids and renal vasa recta
- PMID: 25375646
- PMCID: PMC4222947
- DOI: 10.1371/journal.pone.0111762
Efficient uptake of blood-borne BK and JC polyomavirus-like particles in endothelial cells of liver sinusoids and renal vasa recta
Abstract
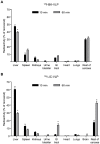
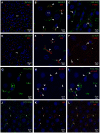
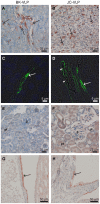
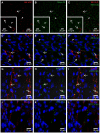
Liver sinusoidal endothelial cells (LSECs) are specialized scavenger cells that mediate high-capacity clearance of soluble waste macromolecules and colloid material, including blood-borne adenovirus. To explore if LSECs function as a sink for other viruses in blood, we studied the fate of virus-like particles (VLPs) of two ubiquitous human DNA viruses, BK and JC polyomavirus, in mice. Like complete virions, VLPs specifically bind to receptors and enter cells, but unlike complete virions, they cannot replicate. 125I-labeled VLPs were used to assess blood decay, organ-, and hepatocellular distribution of ligand, and non-labeled VLPs to examine cellular uptake by immunohisto- and -cytochemistry. BK- and JC-VLPs rapidly distributed to liver, with lesser uptake in kidney and spleen. Liver uptake was predominantly in LSECs. Blood half-life (∼1 min), and tissue distribution of JC-VLPs and two JC-VLP-mutants (L55F and S269F) that lack sialic acid binding affinity, were similar, indicating involvement of non-sialic acid receptors in cellular uptake. Liver uptake was not mediated by scavenger receptors. In spleen, the VLPs localized to the red pulp marginal zone reticuloendothelium, and in kidney to the endothelial lining of vasa recta segments, and the transitional epithelium of renal pelvis. Most VLP-positive vessels in renal medulla did not express PV-1/Meca 32, suggesting location to the non-fenestrated part of vasa recta. The endothelial cells of these vessels also efficiently endocytosed a scavenger receptor ligand, formaldehyde-denatured albumin, suggesting high endocytic activity compared to other renal endothelia. We conclude that LSECs very effectively cleared a large fraction of blood-borne BK- and JC-VLPs, indicating a central role of these cells in early removal of polyomavirus from the circulation. In addition, we report the novel finding that a subpopulation of endothelial cells in kidney, the main organ of polyomavirus persistence, showed selective and rapid uptake of VLPs, suggesting a role in viremic organ tropism.
Conflict of interest statement
Figures




References
-
- Alemany R, Suzuki K, Curiel DT (2000) Blood clearance rates of adenovirus type 5 in mice. J Gen Virol 81: 2605–2609. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

