Neuropathic ocular pain: an important yet underevaluated feature of dry eye
- PMID: 25376119
- PMCID: PMC4366454
- DOI: 10.1038/eye.2014.263
Neuropathic ocular pain: an important yet underevaluated feature of dry eye
Abstract
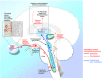
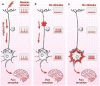
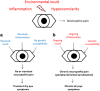
Dry eye has gained recognition as a public health problem given its prevalence, morbidity, and cost implications. Dry eye can have a variety of symptoms including blurred vision, irritation, and ocular pain. Within dry eye-associated ocular pain, some patients report transient pain whereas others complain of chronic pain. In this review, we will summarize the evidence that chronicity is more likely to occur in patients with dysfunction in their ocular sensory apparatus (ie, neuropathic ocular pain). Clinical evidence of dysfunction includes the presence of spontaneous dysesthesias, allodynia, hyperalgesia, and corneal nerve morphologic and functional abnormalities. Both peripheral and central sensitizations likely play a role in generating the noted clinical characteristics. We will further discuss how evaluating for neuropathic ocular pain may affect the treatment of dry eye-associated chronic pain.
Figures

References
-
- The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5 (2:75–92. - PubMed
-
- Stern ME, Gao J, Siemasko KF, Beuerman RW, Pflugfelder SC. The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp Eye Res. 2004;78 (3:409–416. - PubMed
-
- Begley CG, Chalmers RL, Mitchell GL, Nichols KK, Caffery B, Simpson T, et al. Characterization of ocular surface symptoms from optometric practices in North America. Cornea. 2001;20 (6:610–618. - PubMed
-
- Schein OD, Munoz B, Tielsch JM, Bandeen-Roche K, West S. Prevalence of dry eye among the elderly. Am J Ophthalmol. 1997;124 (6:723–728. - PubMed
-
- Moss SE, Klein R, Klein BE. Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol. 2000;118 (9:1264–1268. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

