EGFR mutation testing practices within the Asia Pacific region: results of a multicenter diagnostic survey
- PMID: 25376513
- PMCID: PMC4342317
- DOI: 10.1097/JTO.0000000000000422
EGFR mutation testing practices within the Asia Pacific region: results of a multicenter diagnostic survey
Abstract
Introduction: The efficacy of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors in EGFR mutation-positive non-small-cell lung cancer (NSCLC) patients necessitates accurate, timely testing. Although EGFR mutation testing has been adopted by many laboratories in Asia, data are lacking on the proportion of NSCLC patients tested in each country, and the most commonly used testing methods.
Methods: A retrospective survey of records from NSCLC patients tested for EGFR mutations during 2011 was conducted in 11 Asian Pacific countries at 40 sites that routinely performed EGFR mutation testing during that period. Patient records were used to complete an online questionnaire at each site.
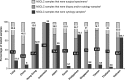
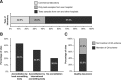
Results: Of the 22,193 NSCLC patient records surveyed, 31.8% (95% confidence interval: 31.2%-32.5%) were tested for EGFR mutations. The rate of EGFR mutation positivity was 39.6% among the 10,687 cases tested. The majority of samples were biopsy and/or cytology samples (71.4%). DNA sequencing was the most commonly used testing method accounting for 40% and 32.5% of tissue and cytology samples, respectively. A pathology report was available only to 60.0% of the sites, and 47.5% were not members of a Quality Assurance Scheme.
Conclusions: In 2011, EGFR mutation testing practices varied widely across Asia. These data provide a reference platform from which to improve the molecular diagnosis of NSCLC, and EGFR mutation testing in particular, in Asia.
Conflict of interest statement
Disclosure: The survey was funded by AstraZeneca. Yasushi Yatabe, Keith Kerr, Teh-Ying Chou, Jabed Iqbal, Jin-Haeng Chung, Koichi Hagiwara, Zhiyong Liang, Nicola Normanno, Keunchil Park, Shinichi Toyooka, Chun-Ming Tsai, Paul Waring, Li Zhang and Tony Mok previously received consulting fees or honoraria from AstraZeneca as well as support for travel to the 2012 expert opinion meeting described in the manuscript. Yasushi Yatabe has previously received consultancy fees from AstraZeneca, Novartis, Roche and Pfizer and payment for lectures from AstraZeneca, Novartis, Roche, Pfizer, Eli Lilly and Abbott Molecular. Keith Kerr has current consultancy agreements with and currently receives payment for lectures from AstraZeneca, Roche, Pfizer, Eli Lilly, Novartis, Merck Serono, Boehringer Ingelheim, and GSK. Ahmad Utomo, Xiang Du and Geon Kook Lee have no conflicts of interest to declare. Ma. Luisa Enriquez’s institution was the recipient of a previous research grant from AstraZeneca and in the past she received support for travel from AstraZeneca to attend an oncology meeting. Jabed Iqbal’s institution currently receives funding from Astra Zeneca for this study. Pathmanathan Rajadurai has an ongoing consultancy agreement with AstraZeneca and also receives payment for local and international CME activities from AstraZeneca. Li Zhang’s institution receives an ongoing grant from AstraZeneca and Roche related to this study, and previously received honoraria from AstraZeneca, Boehringer Ingelheim and Roche; he has also previously received payment for board membership from Eli Lilly and Pfizer, and honoraria from Novartis and Eli Lilly. Van Khanh Tran’s institution is the recipient of an ongoing grant from AstraZeneca and Roche. Shanop Shuangshoti’s institution is the recipient of a previous grant from AstraZeneca. Shinichi Toyooka previously received lecture fees from Taiho Pharmaceutical Co, Sanofi K.K. and Chugai Pharmaceutical Co., Ltd. Keunchil Park receives ongoing consultancy fees from Eli Lilly, Roche, KyowaHakkoKirin, Novartis, Astellas, Clovis and previously received payment for lectures from Eli Lilly. Nicola Normanno’s institution previously received a grant from AstraZeneca to support research activity on
Figures

References
-
- GLOBOCAN (IARC) 2012. Lung cancer fact sheet. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. Accessed March 17, 2014.
-
- Pao W, Girard N. New driver mutations in non-small-cell lung cancer. Lancet Oncol. 2011;12:175–180. - PubMed
-
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

