Phase II clinical trial of pasireotide long-acting repeatable in patients with metastatic neuroendocrine tumors
- PMID: 25376618
- PMCID: PMC4643672
- DOI: 10.1530/ERC-14-0360
Phase II clinical trial of pasireotide long-acting repeatable in patients with metastatic neuroendocrine tumors
Abstract
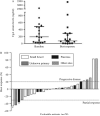
Pasireotide long-acting repeatable (LAR) is a novel somatostatin analog (SSA) with avid binding affinity to somatostatin receptor subtypes 1, 2, 3 (SSTR1,2,3) and 5 (SSTR5). Results from preclinical studies indicate that pasireotide can inhibit neuroendocrine tumor (NET) growth more robustly than octreotide in vitro. This open-label, phase II study assessed the clinical activity of pasireotide in treatment-naïve patients with metastatic grade 1 or 2 NETs. Patients with metastatic pancreatic and extra-pancreatic NETs were treated with pasireotide LAR (60 mg every 4 weeks). Previous systemic therapy, including octreotide and lanreotide, was not permitted. Tumor assessments were performed every 3 months using Response Evaluation Criteria in Solid Tumors (RECIST) criteria. The primary endpoint was progression-free survival (PFS). The secondary endpoints included overall survival (OS), overall radiographic response rate (ORR), and safety. Twenty-nine patients were treated with pasireotide LAR (60 mg every 4 weeks) and 28 were evaluable for response. The median PFS was 11 months. The most favorable effect was observed in patients with low hepatic tumor burden, normal baseline chromogranin A, and high tumoral SSTR5 expression. Median OS has not been reached; the 30-month OS rate was 70%. The best radiographic response was partial response in one patient (4%), stable disease in 17 patients (60%), and progressive disease in ten patients (36%). Although grade 3/4 toxicities were rare, pasireotide LAR treatment was associated with a 79% rate of hyperglycemia including 14% grade 3 hyperglycemia. Although pasireotide appears to be an effective antiproliferative agent in the treatment of advanced NETs, the high incidence of hyperglycemia raises concerns regarding its suitability as a first-line systemic agent in unselected patients. SSTR5 expression is a potentially predictive biomarker for response.
Trial registration: ClinicalTrials.gov NCT01253161.
Keywords: carcinoid; neuroendocrine tumors; pasireotide; somatostatin analogs; somatostatin receptors.
© 2015 Society for Endocrinology.
Figures

References
-
- Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, Cadiot G, Wolin EM, Capdevila J, Wall L, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. New England Journal of Medicine. 2014;371:224–233. doi:10.1056/NEJMoa1316158. - PubMed
-
- Colao A, Petersenn S, Newell-Price J, Findling JW, Gu F, Maldonado M, Schoenherr U, Mills D, Salgado LR, Biller BM, et al. A 12-month phase 3 study of pasireotide in Cushing's disease. New England Journal of Medicine. 2012;366:914–924. doi:10.1056/NEJMoa1105743. - PubMed
-
- Dietrich H, Hu K, Ruffin M, Song D, Bouillaud E, Wang Y, Hasskarl J. Safety, tolerability, and pharmacokinetics of a single dose of pasireotide long-acting release in healthy volunteers: a single-center Phase I study. European Journal of Endocrinology. 2012;166:821–828. doi:10.1530/EJE-11-0773. - PubMed
-
- Faiss S, Pape UF, Böhmig M, Dörffel Y, Mansmann U, Golder W, Riecken EO, Wiedenmann B, International Lanreotide and Interferon Alfa Study Group Prospective, randomized, multicenter trial on the antiproliferative effect of lanreotide, interferon alfa, and their combination for therapy of metastatic neuroendocrine gastroenteropancreatic tumors – the International Lanreotide and Interferon Alfa Study Group. Journal of Clinical Oncology. 2003;21:2689–2696. doi:10.1200/JCO.2003.12.142. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

