Endothelin-a receptor antagonism after renal angioplasty enhances renal recovery in renovascular disease
- PMID: 25377076
- PMCID: PMC4413765
- DOI: 10.1681/ASN.2014040323
Endothelin-a receptor antagonism after renal angioplasty enhances renal recovery in renovascular disease
Abstract
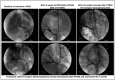
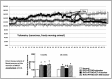
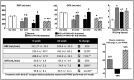
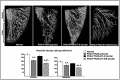
Percutaneous transluminal renal angioplasty/stenting (PTRAS) is frequently used to treat renal artery stenosis and renovascular disease (RVD); however, renal function is restored in less than one half of the cases. This study was designed to test a novel intervention that could refine PTRAS and enhance renal recovery in RVD. Renal function was quantified in pigs after 6 weeks of chronic RVD (induced by unilateral renal artery stenosis), established renal damage, and hypertension. Pigs with RVD then underwent PTRAS and were randomized into three groups: placebo (RVD+PTRAS), chronic endothelin-A receptor (ET-A) blockade (RVD+PTRAS+ET-A), and chronic dual ET-A/B blockade (RVD+PTRAS+ET-A/B) for 4 weeks. Renal function was again evaluated after treatments, and then, ex vivo studies were performed on the stented kidney. PTRAS resolved renal stenosis, attenuated hypertension, and improved renal function but did not resolve renal microvascular rarefaction, remodeling, or renal fibrosis. ET-A blocker therapy after PTRAS significantly improved hypertension, microvascular rarefaction, and renal injury and led to greater recovery of renal function. Conversely, combined ET-A/B blockade therapy blunted the therapeutic effects of PTRAS alone or PTRAS followed by ET-A blockade. These data suggest that ET-A receptor blockade therapy could serve as a coadjuvant intervention to enhance the outcomes of PTRAS in RVD. These results also suggest that ET-B receptors are important for renal function in RVD and may contribute to recovery after PTRAS. Using clinically available compounds and techniques, our results could contribute to both refinement and design of new therapeutic strategies in chronic RVD.
Keywords: angioplasty; endothelin-1; imaging; microcirculation; renal artery stenosis.
Copyright © 2015 by the American Society of Nephrology.
Figures

References
-
- Safian RD, Madder RD: Refining the approach to renal artery revascularization. JACC Cardiovasc Interv 2: 161–174, 2009 - PubMed
-
- Garovic VD, Textor SC: Renovascular hypertension and ischemic nephropathy. Circulation 112: 1362–1374, 2005 - PubMed
-
- White CJ, Olin JW: Diagnosis and management of atherosclerotic renal artery stenosis: Improving patient selection and outcomes. Nat Clin Pract Cardiovasc Med 6: 176–190, 2009 - PubMed
-
- Textor SC: Ischemic nephropathy: Where are we now? J Am Soc Nephrol 15: 1974–1982, 2004 - PubMed
-
- Chade AR, Best PJ, Rodriguez-Porcel M, Herrmann J, Zhu X, Sawamura T, Napoli C, Lerman A, Lerman LO: Endothelin-1 receptor blockade prevents renal injury in experimental hypercholesterolemia. Kidney Int 64: 962–969, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

