Development of apixaban: a novel anticoagulant for prevention of stroke in patients with atrial fibrillation
- PMID: 25377080
- PMCID: PMC4260137
- DOI: 10.1111/nyas.12567
Development of apixaban: a novel anticoagulant for prevention of stroke in patients with atrial fibrillation
Abstract
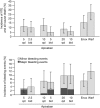
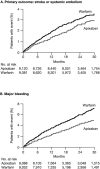
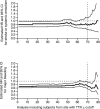
The factor Xa inhibitor apixaban is one of the novel anticoagulants to emerge as alternatives to long-standing standards of care that include low-molecular-weight heparin and warfarin. The development of apixaban reflects a strategy to optimize the clinical pharmacology profile, dosing posology, trial designs, and statistical analyses across multiple indications, and to seek alignment with global health authorities. The primary objective of dose selection was to maintain balance between efficacy and bleeding risk. Twice-daily dosing of apixaban, rather than once daily, was chosen to lower peak concentrations and reduce fluctuations between peak and trough levels. Our discussion here focuses on the use of apixaban for stroke prevention in nonvalvular atrial fibrillation (NVAF). Supporting this indication, a pair of registrational trials was conducted that enrolled the full spectrum of patients who, by guidelines, were eligible for anticoagulation. In the AVERROES study of patients who were unsuitable for warfarin therapy, apixaban was superior to aspirin in reducing the risk of stroke or systemic embolism (SSE), without a significant increase in major bleeding (MB). In the ARISTOTLE (Apixaban for Reduction In STroke and Other ThromboemboLic Events in Atrial Fibrillation) study, apixaban was superior to warfarin on the rates of SSE, MB, and all-cause mortality. Overall, these studies have demonstrated a substantially favorable benefit-risk profile for apixaban over warfarin and aspirin in NVAF.
Keywords: anticoagulation; apixaban; atrial fibrillation; factor Xa inhibition; stroke prevention.
© 2014 The Authors. Annals of the New York Academy of Sciences published by Wiley Periodicals Inc. on behalf of The New York Academy of Sciences.
Figures

References
-
- Hart RG, Pearce LA. Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann. Intern. Med. 2007;146:857–867. - PubMed
-
- Singer DE, Albers GW. Dalen JE American College of Chest Physicians. Antithrombotic therapy in atrial fibrillation: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133(6 Suppl):546S–592S. - PubMed
-
- Harker LA, Hanson SR. Kelly AB. Antithrombotic benefits and hemorrhagic risks of direct thrombin antagonists. Thromb. Haemost. 1995;74:464–472. - PubMed
-
- Morishima Y, Tanabe K, Terada Y, et al. Antithrombotic and hemorrhagic effects of DX-9065a, a direct and selective factor Xa inhibitor: comparison with a direct thrombin inhibitor and antithrombin III-dependent anticoagulants. Thromb. Haemost. 1997;78:1366–1371. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

