TGFβ signaling inhibits goblet cell differentiation via SPDEF in conjunctival epithelium
- PMID: 25377551
- PMCID: PMC4302933
- DOI: 10.1242/dev.117804
TGFβ signaling inhibits goblet cell differentiation via SPDEF in conjunctival epithelium
Abstract
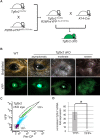
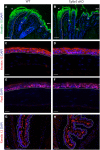
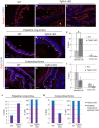
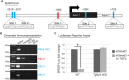
The ocular surface epithelia, including the stratified but non-keratinized corneal, limbal and conjunctival epithelium, in concert with the epidermal keratinized eyelid epithelium, function together to maintain eye health and vision. Abnormalities in cellular proliferation or differentiation in any of these surface epithelia are central in the pathogenesis of many ocular surface disorders. Goblet cells are important secretory cell components of various epithelia, including the conjunctiva; however, mechanisms that regulate goblet cell differentiation in the conjunctiva are not well understood. Herein, we report that conditional deletion of transforming growth factor β receptor II (Tgfbr2) in keratin 14-positive stratified epithelia causes ocular surface epithelial hyperplasia and conjunctival goblet cell expansion that invaginates into the subconjunctival stroma in the mouse eye. We found that, in the absence of an external phenotype, the ocular surface epithelium develops properly, but young mice displayed conjunctival goblet cell expansion, demonstrating that TGFβ signaling is required for normal restriction of goblet cells within the conjunctiva. We observed increased expression of SAM-pointed domain containing ETS transcription factor (SPDEF) in stratified conjunctival epithelial cells in Tgfbr2 cKO mice, suggesting that TGFβ restricted goblet cell differentiation directly by repressing Spdef transcription. Gain of function of Spdef in keratin 14-positive epithelia resulted in the ectopic formation of goblet cells in the eyelid and peripheral cornea in adult mice. We found that Smad3 bound two distinct sites on the Spdef promoter and that treatment of keratin 14-positive cells with TGFβ inhibited SPDEF activation, thereby identifying a novel mechanistic role for TGFβ in regulating goblet cell differentiation.
Keywords: Conjunctiva; Differentiation; Goblet cells; Mouse; SPDEF; TGFβ signaling.
© 2014. Published by The Company of Biologists Ltd.
Figures





Similar articles
-
Spdef null mice lack conjunctival goblet cells and provide a model of dry eye.Am J Pathol. 2013 Jul;183(1):35-48. doi: 10.1016/j.ajpath.2013.03.017. Epub 2013 May 10. Am J Pathol. 2013. PMID: 23665202 Free PMC article.
-
SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production.J Clin Invest. 2009 Oct;119(10):2914-24. doi: 10.1172/JCI39731. Epub 2009 Sep 14. J Clin Invest. 2009. PMID: 19759516 Free PMC article.
-
Goblet cells of the conjunctiva: A review of recent findings.Prog Retin Eye Res. 2016 Sep;54:49-63. doi: 10.1016/j.preteyeres.2016.04.005. Epub 2016 Apr 16. Prog Retin Eye Res. 2016. PMID: 27091323 Free PMC article. Review.
-
Goblet Cells Contribute to Ocular Surface Immune Tolerance-Implications for Dry Eye Disease.Int J Mol Sci. 2017 May 5;18(5):978. doi: 10.3390/ijms18050978. Int J Mol Sci. 2017. PMID: 28475124 Free PMC article.
-
Three cheers for the goblet cell: maintaining homeostasis in mucosal epithelia.Trends Mol Med. 2015 Aug;21(8):492-503. doi: 10.1016/j.molmed.2015.06.003. Epub 2015 Jul 3. Trends Mol Med. 2015. PMID: 26144290 Review.
Cited by
-
Yap/Taz inhibit goblet cell fate to maintain lung epithelial homeostasis.Cell Rep. 2021 Jul 13;36(2):109347. doi: 10.1016/j.celrep.2021.109347. Cell Rep. 2021. PMID: 34260916 Free PMC article.
-
Blocking TGF-β and β-Catenin Epithelial Crosstalk Exacerbates CKD.J Am Soc Nephrol. 2017 Dec;28(12):3490-3503. doi: 10.1681/ASN.2016121351. Epub 2017 Jul 12. J Am Soc Nephrol. 2017. PMID: 28701516 Free PMC article.
-
Chemical conversion of human epidermal stem cells into intestinal goblet cells for modeling mucus-microbe interaction and therapy.Sci Adv. 2021 Apr 14;7(16):eabb2213. doi: 10.1126/sciadv.abb2213. Print 2021 Apr. Sci Adv. 2021. PMID: 33853767 Free PMC article.
-
TSP50 Attenuates DSS-Induced Colitis by Regulating TGF-β Signaling Mediated Maintenance of Intestinal Mucosal Barrier Integrity.Adv Sci (Weinh). 2024 Mar;11(11):e2305893. doi: 10.1002/advs.202305893. Epub 2024 Jan 8. Adv Sci (Weinh). 2024. PMID: 38189580 Free PMC article.
-
Characterization of doxycycline-dependent inducible Simian Virus 40 large T antigen immortalized human conjunctival epithelial cell line.PLoS One. 2019 Sep 11;14(9):e0222454. doi: 10.1371/journal.pone.0222454. eCollection 2019. PLoS One. 2019. PMID: 31509592 Free PMC article.
References
-
- Barsky S., Siegal G., Jannotta F. and Liotta L. (1983). Loss of basement membrane components by invasive tumors but not by their benign counterparts. Lab. Invest. 49, 140-147. - PubMed
-
- Bian Y., Terse A., Du J., Hall B., Molinolo A., Zhang P., Chen W., Flanders K. C., Gutkind J. S., Wakefield L. M. et al. (2009). Progressive tumor formation in mice with conditional deletion of TGF-β signaling in head and neck epithelia is associated with activation of the PI3K/Akt pathway. Cancer Res. 69, 5918-5926 10.1158/0008-5472.CAN-08-4623 - DOI - PMC - PubMed
-
- Bleuming S. A., He X. C., Kodach L. L., Hardwick J. C., Koopman F. A., ten Kate F. J., van Deventer S. J. H., Hommes D. W., Peppelenbosch M. P., Offerhaus G. J. et al. (2007). Bone morphogenetic protein signaling suppresses tumorigenesis at gastric epithelial transition zones in mice. Cancer Res. 67, 8149-8155 10.1158/0008-5472.CAN-06-4659 - DOI - PubMed
-
- Chen G., Korfhagen T. R., Xu Y., Kitzmiller J., Wert S. E., Maeda Y., Gregorieff A., Clevers H. and Whitsett J. A. (2009). SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J. Clin. Invest. 119, 2914-2924. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

