Fine-mapping of the HNF1B multicancer locus identifies candidate variants that mediate endometrial cancer risk
- PMID: 25378557
- PMCID: PMC4321445
- DOI: 10.1093/hmg/ddu552
Fine-mapping of the HNF1B multicancer locus identifies candidate variants that mediate endometrial cancer risk
Abstract
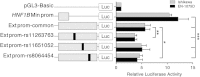
Common variants in the hepatocyte nuclear factor 1 homeobox B (HNF1B) gene are associated with the risk of Type II diabetes and multiple cancers. Evidence to date indicates that cancer risk may be mediated via genetic or epigenetic effects on HNF1B gene expression. We previously found single-nucleotide polymorphisms (SNPs) at the HNF1B locus to be associated with endometrial cancer, and now report extensive fine-mapping and in silico and laboratory analyses of this locus. Analysis of 1184 genotyped and imputed SNPs in 6608 Caucasian cases and 37 925 controls, and 895 Asian cases and 1968 controls, revealed the best signal of association for SNP rs11263763 (P = 8.4 × 10(-14), odds ratio = 0.86, 95% confidence interval = 0.82-0.89), located within HNF1B intron 1. Haplotype analysis and conditional analyses provide no evidence of further independent endometrial cancer risk variants at this locus. SNP rs11263763 genotype was associated with HNF1B mRNA expression but not with HNF1B methylation in endometrial tumor samples from The Cancer Genome Atlas. Genetic analyses prioritized rs11263763 and four other SNPs in high-to-moderate linkage disequilibrium as the most likely causal SNPs. Three of these SNPs map to the extended HNF1B promoter based on chromatin marks extending from the minimal promoter region. Reporter assays demonstrated that this extended region reduces activity in combination with the minimal HNF1B promoter, and that the minor alleles of rs11263763 or rs8064454 are associated with decreased HNF1B promoter activity. Our findings provide evidence for a single signal associated with endometrial cancer risk at the HNF1B locus, and that risk is likely mediated via altered HNF1B gene expression.
© The Author 2014. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures



References
-
- Tavassoli F.A., Stratton M.R. Tumors of the Breast and Female Genital Organs. Lyon: IARC Press; 2003.
-
- Gudmundsson J., Sulem P., Steinthorsdottir V., Bergthorsson J.T., Thorleifsson G., Manolescu A., Rafnar T., Gudbjartsson D., Agnarsson B.A., Baker A., et al. Two variants on chromosome 17 confer prostate cancer risk, and the one in TCF2 protects against type 2 diabetes. Nat. Genet. 2007;39:977–983. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA64277/CA/NCI NIH HHS/United States
- CA128978/CA/NCI NIH HHS/United States
- 068545/Z/02/WT_/Wellcome Trust/United Kingdom
- 16565/CRUK_/Cancer Research UK/United Kingdom
- CAPMC/ CIHR/Canada
- C12292/A11174/CRUK_/Cancer Research UK/United Kingdom
- 16459/CRUK_/Cancer Research UK/United Kingdom
- R01 CA128978/CA/NCI NIH HHS/United States
- R01 CA90899/CA/NCI NIH HHS/United States
- C1287/A12014/CRUK_/Cancer Research UK/United Kingdom
- 16563/CRUK_/Cancer Research UK/United Kingdom
- C1281/A12014/CRUK_/Cancer Research UK/United Kingdom
- UM1 CA182910/CA/NCI NIH HHS/United States
- 1U19 CA148065/CA/NCI NIH HHS/United States
- C5047/A10692/CRUK_/Cancer Research UK/United Kingdom
- R01 CA092585/CA/NCI NIH HHS/United States
- 1U19 CA148537/CA/NCI NIH HHS/United States
- R01 CA090899/CA/NCI NIH HHS/United States
- C490/A10124/CRUK_/Cancer Research UK/United Kingdom
- C5047/A8384/CRUK_/Cancer Research UK/United Kingdom
- C1287/A 10710/CRUK_/Cancer Research UK/United Kingdom
- R01 CA064277/CA/NCI NIH HHS/United States
- U19 CA148537/CA/NCI NIH HHS/United States
- R01 CA148667/CA/NCI NIH HHS/United States
- 090532/WT_/Wellcome Trust/United Kingdom
- R01 CA122443/CA/NCI NIH HHS/United States
- P30 CA015083/CA/NCI NIH HHS/United States
- C5047/A15007/CRUK_/Cancer Research UK/United Kingdom
- 1U19 CA148112/CA/NCI NIH HHS/United States
- G0000934/MRC_/Medical Research Council/United Kingdom
- R01 CA 092585/CA/NCI NIH HHS/United States
- U19 CA148112/CA/NCI NIH HHS/United States
- U19 CA148065/CA/NCI NIH HHS/United States
- 085475/WT_/Wellcome Trust/United Kingdom
- C1287/A10118/CRUK_/Cancer Research UK/United Kingdom
- P50 CA136393/CA/NCI NIH HHS/United States
- 16561/CRUK_/Cancer Research UK/United Kingdom
- 10124/CRUK_/Cancer Research UK/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

