CD4 T cells specific for a latency-associated γ-herpesvirus epitope are polyfunctional and cytotoxic
- PMID: 25378595
- PMCID: PMC4301266
- DOI: 10.4049/jimmunol.1302060
CD4 T cells specific for a latency-associated γ-herpesvirus epitope are polyfunctional and cytotoxic
Abstract
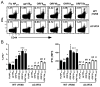
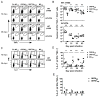
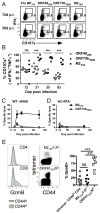
The oncogenic γ-herpesviruses EBV and Kaposi sarcoma-associated herpesvirus are ubiquitous human pathogens that establish lifelong latent infections maintained by intermittent viral reactivation and reinfection. Effector CD4 T cells are critical for control of viral latency and in immune therapies for virus-associated tumors. In this study, we exploited γHV68 infection of mice to enhance our understanding of the CD4 T cell response during γ-herpesvirus infection. Using a consensus prediction approach, we identified 16 new CD4 epitope-specific responses that arise during lytic infection. An additional epitope encoded by the M2 protein induced uniquely latency-associated CD4 T cells, which were not detected at the peak of lytic infection but only during latency and were not induced postinfection with a latency-deficient virus. M2-specific CD4 T cells were selectively cytotoxic, produced multiple antiviral cytokines, and sustained IL-2 production. Identification of latency-associated cytolytic CD4 T cells will aid in dissecting mechanisms of CD4 immune control of γ-herpesvirus latency and the development of therapeutic approaches to control viral reactivation and pathology.
Copyright © 2014 by The American Association of Immunologists, Inc.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures

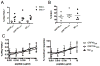
References
-
- Blackman MA, Flano E, Usherwood E, Woodland DL. Murine gamma-herpesvirus-68: a mouse model for infectious mononucleosis? Mol Med Today. 2000;6:488–490. - PubMed
-
- Martorelli D, Muraro E, Merlo A, Turrini R, Rosato A, Dolcetti R. Role of CD4+ cytotoxic T lymphocytes in the control of viral diseases and cancer. Int Rev Immunol. 2010;29:371–402. - PubMed
-
- Paludan C, Munz C. CD4+ T cell responses in the immune control against latent infection by Epstein-Barr virus. Curr Mol Med. 2003;3:341–347. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HHSN272200900044C/AI/NIAID NIH HHS/United States
- CA148250/CA/NCI NIH HHS/United States
- R01 CA168558/CA/NCI NIH HHS/United States
- HHSN272200900042C/AI/NIAID NIH HHS/United States
- CA168558/CA/NCI NIH HHS/United States
- P30 CA046934/CA/NCI NIH HHS/United States
- T32AI049823/AI/NIAID NIH HHS/United States
- T32 AI049823/AI/NIAID NIH HHS/United States
- R01 AI042927/AI/NIAID NIH HHS/United States
- HHSN272200900044C/AI/NIAID NIH HHS/United States
- AI082919/AI/NIAID NIH HHS/United States
- R21 AI082919/AI/NIAID NIH HHS/United States
- RC2 CA148250/CA/NCI NIH HHS/United States
- AI42927/AI/NIAID NIH HHS/United States
- F32AI084327/AI/NIAID NIH HHS/United States
- HHSN272201300006C/AI/NIAID NIH HHS/United States
- F32 AI084327/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

